INTRODUCTION
Full-arch dental implant reconstruction is a viable treatment choice for patients who are edentulous or who have teeth that are compromised and in need of extraction. Regardless of a free-hand or fully guided surgical protocol, treatment outcomes for full-arch, implant-supported restorations have helped patients regain proper function, aesthetics, and quality of life.1-3 Additionally, the ability to place implants immediately after tooth extraction has become a viable treatment modality that can often reduce the time needed to deliver functional restorations.4 However, the residual alveolar ridge may require grafting to fill defects left by extraction sockets or pre-existing concavities.5,6 It is well understood that substantial bone resorption and loss of bone volume can occur when extraction sites are not grafted.7,8 Avila-Ortiz et al concluded that “alveolar ridge preservation is an effective therapy to attenuate the dimensional reduction of the alveolar ridge that normally takes place after tooth extraction.”9 The gold standard has always been autologous tissue harvested from the patient, which is not always easy or readily accessible. Therefore, most clinicians currently utilize bone and membranes available through tissue banks. Current innovations, however, have fortunately provided a new, previously untapped source for this autologous tissue: the extracted tooth, which is often readily available when full-arch implant reconstruction is planned. This current article will demonstrate that it is possible to provide enough grafting material volume to fill all residual sockets and concavities from extracted teeth harvested during immediate implant placement for a dual-arch surgical procedure.
CASE REPORT
A 68-year-old female presented with failing dentition in the maxillary and mandibular arches due to years of neglect and patchwork dentistry. The patient was unhappy with the condition of her teeth and was embarrassed to go out in public. She had difficulty chewing due to missing and fractured teeth in the maxillary arch, did not have any posterior mandibular teeth, and did not have a repeatable bite position. The patient had been to several dentists who offered differing treatment plans and was very confused regarding potential options to correct the deficiencies to improve her quality of life. Options that were presented included, but were not limited to, (1) removable partial dentures (RPD), (2) a maxillary complete denture vs a mandibular RPD, and (3) implant-supported removable and fixed restorations for both arches. The patient wished to determine if a fixed-type full-arch restoration could be considered for both the maxilla and mandible. The patient’s medical history revealed hyperthyroidism and hip replacement within the prior 5 years.
Clinical examination confirmed the diminished condition of the patient’s dentition. The need for a thorough 3D assessment of the patient’s existing anatomical presentation, which could only be accomplished with CBCT, was explained to her. The CBCT allowed for the inspection of the anatomy in multiple views and utilizing the digital tools afforded by the software (CS 3D Imaging [Carestream Dental]) (Figure 1). The panoramic reconstruction served as a “scout” film to help visualize the present condition of the patient’s dentition (Figure 2). The upper arch exhibited several fractured teeth, several with previous root canal treatment, one single crown, and a 4-unit posterior bridge on teeth Nos. 12 to 15. Using the embedded link, the original CBCT scan data was then exported from the Carestream 3D Imaging Software directly into Blue Sky Plan software (Blue Sky Bio). The Blue Sky plan offers additional planning and design tools to aid in accurate diagnosis, treatment planning, and surgical guide fabrication.
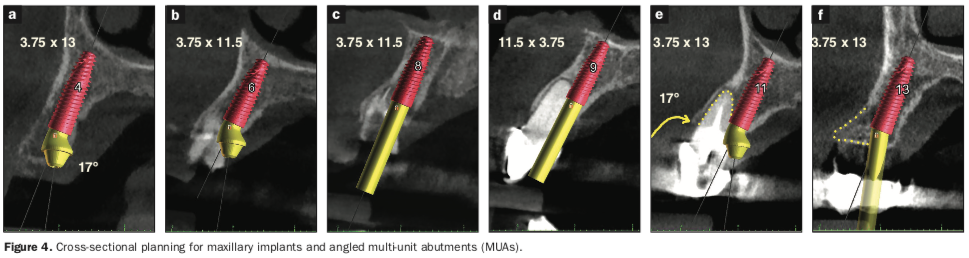
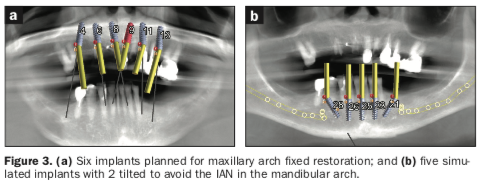
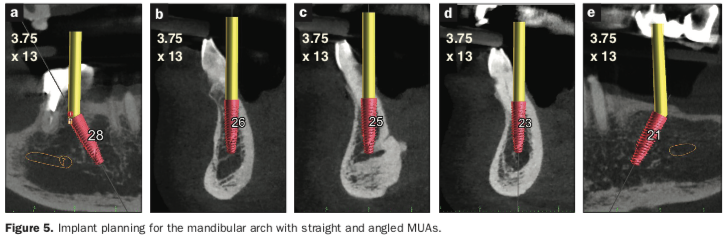
The preliminary plan consisted of placing implants in strategic positions to support fixed, implant-supported restorations that would be accurately delivered with the implementation of static, sequential surgical guides (Figure 3). Each potential implant receptor site was designated by tooth number for the maxillary and mandibular arches. Manufacturer-specific simulated implants were then refined within the cross-sectional images, recording diameters and lengths in screenshots for the maxilla (Figure 4) and the mandible (Figure 5) that were utilized during the surgery as color printouts.
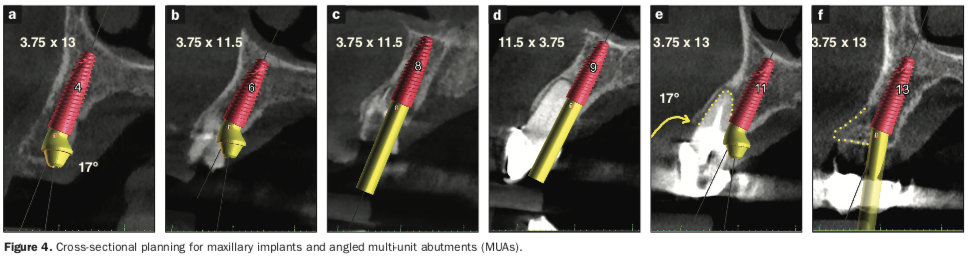
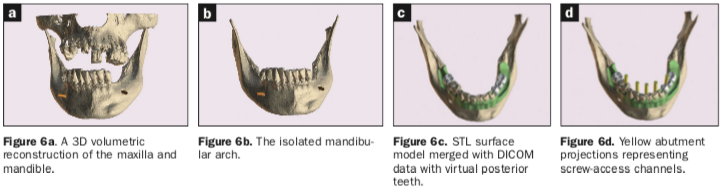
When assessing the potential mandibular implant receptor sites, the buccal and lingual cortical plates appeared to be well-defined. However, careful inspection revealed that a deficient density exhibited within the intermedullary bone. Yellow abutment “projections” represented simulated abutment trajectories helpful in the determination of screw-access channels within the transitional and final prostheses. It was also possible to place realistic simulated abutments based on the desired angulation and tissue cuff height chosen from the implant library within the software (Figures 4a and 4b). The planning continued with the examination and manipulation of the 3D reconstructed volume of the mandible and maxilla (Figure 6a). Using the “isolate” function within the Blue Sky Plan software, the mandibular arch was separated from the maxillary arch, which, with the merging of the intraoral scanning data, helped with the restoratively driven planning and refinement of implant positioning (Figures 6b and 6c). The implants were then planned with precise regard for the emergence of the screw-access channels represented by the yellow abutment projections, which extended above the occlusal plane (Figure 6d).
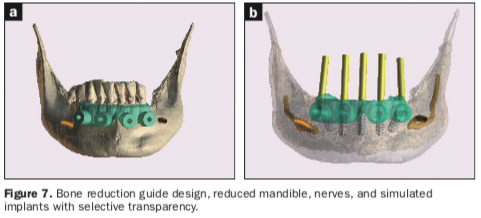
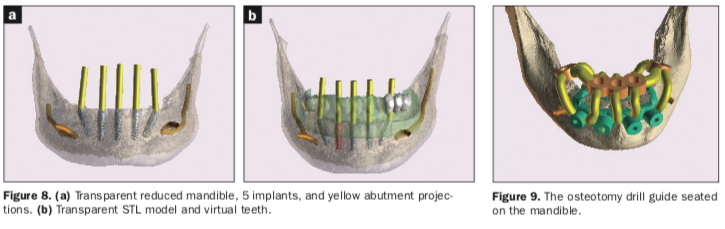
Once each of the implant receptor sites and the vertical positions were validated, the amount of alveolar reduction (after tooth extraction) was determined. A bone reduction guide was then designed with 4 anchor pins for stable fixation to the mandible (Figure 7a). The various components of the diagnostic progress can be better appreciated using “selective transparency” to visualize structures based on their densities (Figure 7b). Selective transparency was again utilized to visualize the final location of the 3 central straight implants and the 2 angled implants clearly indicating the safe proximity to the bilateral inferior alveolar nerves (Figure 8a). The translucent STL model of the mandibular teeth and virtual teeth helped relate the implant positions for the virtual restorative plan (Figure 8b). The sequential osteotomy drill guide was designed based upon the parameters of the implant system and guided drill kit utilized. The osteotomy drill guide was to be secured to the mandible with the same fixation pins as the bone reduction guide (Figure 9).
Clinical Presentation
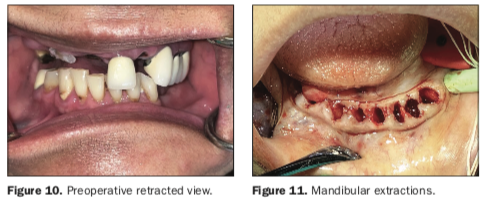
The patient presented with a collapsed bite due to missing, mobile, and fractured teeth, which severely affected her ability to masticate food, resulting in embarrassment and a diminished quality of life (Figure 10). After a thorough review of the diagnostic process, the treatment plan was presented and accepted by the patient for maxillary and mandibular implant-supported, fixed restorations. At the request of the patient, one long procedure was scheduled to be completed under sedation administered by a dental anesthesiologist. Once the patient had been sedated, bilateral mandibular blocks were accomplished with 2% lidocaine with 1:100,000 epinephrine and 4% articaine. The remaining mandibular teeth were extracted using periotomes, elvatomes, and forceps (TBS Instruments), and all sockets were thoroughly debrided and then irrigated with chlorhexidine gluconate 0.12% (Figure 11). Many of the extracted teeth were free of decay, root canal treatment, or fillings, and it was therefore elected to utilize the patient’s own teeth to fabricate autologous graft material for use in both the maxillary and mandibular arches. The process of harvesting graft material from tooth structure has been successfully reported in the literature and has become a great source of autologous tissue when teeth are to be extracted and grafting is required.
When teeth are to be extracted, the extraction sites and implant receptor sites will often require some type of grafting to manage the resultant anatomical defects and bony concavities. Currently, most bone grafting is dependent on tissue banks to supply us with “bone in a bottle” in various shapes, sizes, and formulations.
While these products are essential to have on hand when teeth are to be extracted, perhaps an alternative concept would be to use the autologous material from enamel and dentin to serve as grafting material to fill defects and augment the surgical sites.
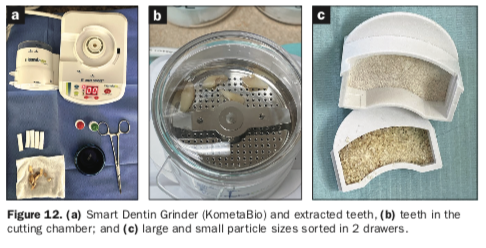
As many of our patients present with a failing dentition due to alveolar bone loss, dentin grinding has gained popularity as an important ancillary method to gain significant volumes of graft material, especially when patients are to undergo full-arch dental implants.10-12 One such innovation is the Smart Dentin Grinder (KometaBio) (Figure 12a).
Once the remaining mandibular teeth were extracted and evaluated, a diamond bur in a high-speed handpiece was used to clean the tooth roots and areas of the enamel, removing all debris, soft-tissue tags, fillings, and decay. The teeth were then dried and placed in the single-use sterile chamber attached to the Smart Dentin Grinder (Figure 12b).
The grinding process was timed for 3 seconds, followed by a 10-second sorting process. It was repeated until the teeth were sufficiently ground and the particles separated and sorted by size within the canister and collection drawers. The particle size ranged from 250 to 1,200 µm as collected in 2 drawers (Figure 12c). The volume of autologous particulate material was impressive at approximately 5 to 6 cc of graft material generated from the extracted teeth.
As per the recommended cleansing protocols, the graft material was transferred from the top and bottom drawer to a sterile dish. The entire volume of graft material was then covered with the dentin cleanser solution and left covered for 5 minutes. The material was dehydrated with a sterile gauze. This liquid cleansing process effectively rendered the dentin particulate bacteria-free without harming the collagen, BMPs, and growth factors embedded in the dentin. A phosphate-buffered saline was then used to neutralize the pH levels, followed by dehydrating with a sterile gauze, and then followed by a repeat of the rinse process. Then it was saved for later use as needed in both the upper and lower arches. The entire process can range from 8 to 10 minutes and is usually completed by a trained auxiliary.
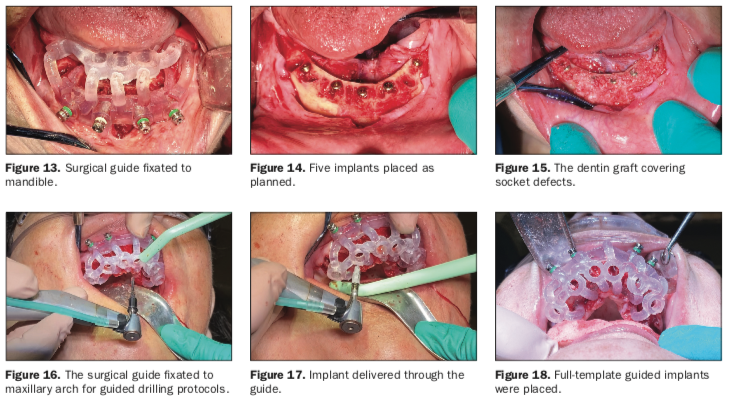
A full-thickness mucoperiosteal flap was elevated from the approximate areas of teeth Nos. 30 to 20 and carefully reflected to expose the alveolar ridge. A bone reduction guide was placed over the site and fixated with 4 anchor pins (not shown). The bone was then reduced to the planned vertical height with rongeurs and flattened with carbide burs in a straight handpiece (Alveoplasty Kit [Meisinger USA]). Based upon the 3D planning, the 3D printed osteotomy drill guide was designed to fit over the reduced bone and fixated in the same holes as the bone reduction guide (Figure 13).
The fixation pins were of 2 different lengths and secured the resin guide to the mandible (Fixation Kit [ROE Dental Laboratory]).
The osteotomies were prepared with sequential guided drills for accuracy, and 5 implants were placed approximately 2 mm subcrestal (Helix GM [Neodent, a Straumann Group Brand]) (Figure 14). Although the implants exhibited moderate insertion torque, the intermedullary bone density within the mandibular implant receptor sites was poor, as previously noted during the diagnostic phase. Each implant was then objectively tested for stability using resonance frequency analysis (RFA), and implant stability quotients (ISQs) were recorded (Osstell IDx [Osstell, a W&H Company]).
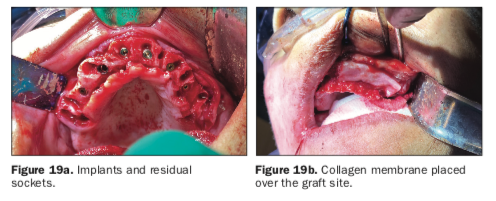
The ISQ values confirmed the initial CBCT assessment of the mandibular bone, and a decision was made to bury the implants for approximately 2 to 3 months to provide sufficient opportunity and time for the implants to fully integrate within the mandibular bone prior to loading. Each subcrestally placed implant received a 2-mm cap screw to fill the coronal osteotomy site. All the residual tooth sockets and any defects or concave areas were then filled with the dentin graft material (Figure 15).
Two 20 × 30 mm collagen membranes (Maxxeus [Community Tissue Services]) were then draped over the grafted site and stabilized with deep horizontal mattress sutures. Closure was then achieved with continuous and interrupted 4-0 sutures (VICRYL [Ethicon]).
A similar procedure was completed for the maxillary arch. After local infiltration of anesthetic agents, all remaining root tips and teeth were atraumatically extracted, and all sockets were thoroughly debrided. A full-thickness mucoperiosteal flap extended from approximately the area of teeth Nos. 3 to 14 to expose the residual alveolus. Once the bone was reduced, an osteotomy drill guide was fixated to the maxillary arch (Figure 16). Osteotomies were then prepared, and 6 Helix GM implants were placed through the guide (Figures 17 and 18).
The stability of each implant was objectively measured, and ISQ values were found to be below the threshold for immediate loading. Therefore, the maxillary implants were buried in a 2-stage protocol. To preserve the width and height of the residual alveolar ridge, the extraction sites were all filled with the graft material gleaned from the teeth removed from the lower arch (Figure 19a) and covered with large 20 × 30 mm collagen membranes (Figure 19b). The immediate postoperative panoramic radiograph revealed the placement of 5 implants for the mandibular arch and 6 for the maxillary arch (Figure 20).
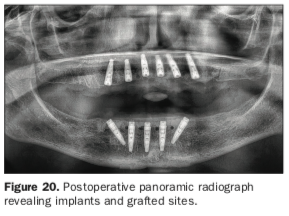
The classic radiolucent appearance of fresh extraction sites was not evident as each was filled with the dentin graft material. Small, round, radiolucent “holes” could be visualized in the mandibular arch from the 4 fixation screws. The 2D panoramic reconstructed view is somewhat distorted, and thus, the true trajectory of each implant cannot be accurately appreciated. It was the original plan that the right- and leftmost distal “tilted” implants receive 30° angulated multi-unit abutments at the appropriate tissue cuff height once the implants were uncovered and after osseointegration had been confirmed. The patient was then brought out of sedation and allowed to recover until she was fully coherent and ambulatory. Immediate complete dentures were then delivered to the patient after soft-tissue relining was accomplished to improve fit. Post-op instructions were provided to the patient orally and in writing. The procedure was well-tolerated, and the patient has been followed for suture removal and healing progress.
DISCUSSION
When full-arch implant restoration is contemplated for patients who are partially dentate, immediate extractions will be required. Many will require extractions of perfectly intact teeth and roots that can provide an excellent source for autologous graft material. These extraction sockets may leave significant voids in the bony architecture of the remaining alveolus. As it is recommended that implants should be planned with 3D imaging acquired through CBCT scans, the diagnosis should also include an assessment of where the teeth will be removed and what type of bony defects will be left after extraction. When implants are planned to be placed directly within a fresh extraction socket, often there is a gap on the buccal, which can be filled with graft material to help preserve the bony housing. In other areas, the whole sockets can be filled to reduce the potential for volumetric shrinkage of the ridge over time.
The current case presentation illustrated the effectiveness of utilizing an innovative device to grind extracted teeth to produce sufficient graft volumes required during the surgical phase of full-arch implant reconstruction. Calvo-Guirado et al found that after processing with the Smart Dentin Grinder, “0.25 gr of human teeth produced 1.0 cc of biomaterial” and that the “chemical composition of the particulate was clearly similar to natural bone.”13 The present case illustrated immediate extractions and immediate implant placements for a delayed loading protocol with autologous dentin graft material, which can also be used for immediate load protocols when appropriate.
CONCLUSION
Full-arch, implant-supported restorations can be either fixed or removable overdentures. Regardless of the proposed treatment modality, when extractions are required, it is recommended that grafting be an integral and necessary part of the surgical procedure. The use of autologous tissue generated from the patient’s own teeth has many advantages, including (1) representing a biocompatible material and not being recognized as a foreign body; (2) having almost the same composition as bone, composed of higher density hydroxyapatite and type I collagen fiber; (3) dentin and enamel that are tougher than cortical bone and, therefore, provide an excellent scaffold, hence osteoconductivity; (4) dentin that contains good amounts of BMPs and growth factors that aid in the regeneration process to form new bone relatively quicker than most grafts, hence osteoinductivity; (5) a single tooth, dependent on the type, that can produce anywhere between 0.5 to 2.5 cc, providing ample amount of graft material; (6) autologous dentin that does not require a secondary harvesting site and, therefore, eliminates morbidity, risk, and pain associated with that secondary procedure; and (7) reducing costs related to purchasing bone grafting material from tissue banks. While dentin grafting can be especially useful with full-arch, implant-supported restorations, additional uses can include (1) conventional socket preservation, (2) onlay grafting, (3) sinus augmentations, (4) creating sticky bone with platelet-rich fibrin, and (5) use with partial extraction/socket shield cases,14 like any other available grafting material. Patients are also pleased that their own cells are being used to enhance the healing process. More research, long-term studies, and follow-up procedures are recommended to quantify the benefits of this adjunct modality to provide autologous grafting material for patients in need.
REFERENCES
1. Morton D, Gallucci G, Lin WS, et al. Group 2 ITI Consensus Report: Prosthodontics and implant dentistry. Clin Oral Implants Res. 2018;29 Suppl 16:215-223. doi:10.1111/clr.13298
2. Lerner H, Hauschild U, Sader R, et al. Complete-arch fixed reconstruction by means of guided surgery and immediate loading: a retrospective clinical study on 12 patients with 1 year of follow-up. BMC Oral Health. 2020;20(1):15. doi:10.1186/s12903-019-0941-z
3. Marra R, Acocella A, Alessandra R, et al. Rehabilitation of full-mouth edentulism: immediate loading of implants inserted with computer-guided flapless surgery versus conventional dentures: a 5-year multicenter retrospective analysis and OHIP questionnaire. Implant Dent. 2017;26(1):54-58. doi:10.1097/ID.0000000000000492
4. Kan JYK, Rungcharassaeng K, Deflorian M, et al. Immediate implant placement and provisionalization of maxillary anterior single implants. Periodontol 2000. 2018;77(1):197-212. doi:10.1111/prd.12212
5. Tan WL, Wong TL, Wong MC, et al. A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin Oral Implants Res. 2012;23 Suppl 5:1-21. doi:10.1111/j.1600-0501.2011.02375.x
6. Schropp L, Wenzel A, Kostopoulos L, et al. Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int J Periodontics Restorative Dent. 2003;23(4):313–23.
7. Chan HL, Lin GH, Fu JH, et al. Alterations in bone quality after socket preservation with grafting materials: a systematic review. Int J Oral Maxillofac Implants. 2013;28(3):710–20. doi:10.11607/jomi.2913
8. Bassir SH, Alhareky M, Wangsrimongkol B, et al. Systematic review and meta-analysis of hard tissue outcomes of alveolar ridge preservation. Int J Oral Maxillofac Implants. 2018;33(5):979-994. doi:10.11607/jomi.6399
9. Avila-Ortiz G, Chambrone L, Vignoletti F. Effect of alveolar ridge preservation interventions following tooth extraction: a systematic review and meta-analysis. J Clin Periodontol. 2019;46 Suppl 21:195-223. doi:10.1111/jcpe.13057
10. Mazor Z, Horowitz RA, Prasad H, et al. Healing dynamics following alveolar ridge preservation with autologous tooth structure. Int J Periodontics Restorative Dent. 2019;39(5):697-702. doi:10.11607/prd.4138
11. Santos A, Botelho J, Machado V, et al. Autogenous mineralized dentin versus xenograft granules in ridge preservation for delayed implantation in post-extraction sites: a randomized controlled clinical trial with an 18 months follow-up. Clin Oral Implants Res. 2021;32(8):905-915. doi:10.1111/clr.13765
12. Simos S. A conservative approach to immediate bone grafting. Dent Today. 2021;40(3):20-23.
13. Calvo-Guirado JL, Ballester-Montilla A, N De Aza P, et al. Particulated, extracted human teeth characterization by SEM⁻EDX evaluation as a biomaterial for socket preservation: an in vitro study. Materials (Basel). 2019;12(3):380. doi:10.3390/ma12030380
14. Pohl S, Binderman I, Božić D, et al. Effectiveness of autologous tissue grafts on soft tissue ingrowth in patients following partial root extraction with socket shield: a retrospective analysis of a case series. Int J Oral Maxillofac Implants. 202;36(2):362-370. doi:10.11607/jomi.8581
ABOUT THE AUTHORS
Dr. Ganz received his dental degree from the University of Medicine and Dentistry of New Jersey and a specialty certificate in maxillofacial prosthetics/prosthodontics at the University of Texas MD Anderson Cancer Center in Houston. He is a Fellow of the Academy of Osseointegration, a Fellow of the International College of Dentists, a Diplomate of the International Congress of Oral Implantologists (ICOI), US Ambassador of the Digital Dental Society, and co-director of Advanced Implant Education (AIE). Dr. Ganz was recently honored by the American Academy of Implant Dentistry and the Digital Dentistry Society for his lifelong contributions. He is on the faculty of the Rutgers School of Dental Medicine, is the director of oral restoration at Park40 in New York City, and maintains a private practice in Fort Lee, NJ. He can be reached at drganz@drganz.com.
Dr. Tawil received his DDS degree from the New York University College of Dentistry and his Master of Biological Sciences degree from Long Island University. He is co-director of AIE. He is a Diplomate of the International Academy of Dental Implantology as well as a Fellow of the ICOI and the Advanced Implant Academy. He has received recognition for outstanding achievement in dental implants from the Advanced Implant Academy and has received the President’s Service Award for his volunteer work. Dr. Tawil lectures internationally on advanced dental implant procedures. He maintains a general private practice in Brooklyn, NY, where he focuses on implant therapy. He can be reached at tawildental@gmail.com.
Disclosures: Dr. Ganz receives lecture honoraria for lectures from ROE Dental Laboratory, Osstell, and Carestream Dental. Dr. Tawil reports no disclosures.