INTRODUCTION
It is globally accepted that 3D cleaning and disinfection are central to endodontic success and that bacteria are ubiquitous in endodontically failing teeth. Yet, there is ongoing controversy regarding the very clinical methods used that directly influence eliminating these microbial invaders. The role of bacteria in the pathogenesis of endodontic disease is well established, and therefore, it is critical to eradicate these pathogens by employing the highest level of presently developed standards.1
The goals of contemporary endodontics are directed toward shaping canals, 3D cleaning, and filling root canal systems—often referred to as the 3 pillars of the endodontic trifecta or triad. It is appreciated that each pillar, individually and in combination, serves to influence the biological objectives for predictably successful endodontics (Figure 1).
Figure 1. This radiographic image demonstrates that shaping canals facilitate the 3D cleaning and filling of root canal systems.
Some proponents of minimally invasive endodontics are advocating minimal instrumentation of a canal or no instrumentation at all. Yet, the question remains, can a minimally prepared canal and its related root canal system be predictably cleaned and filled? From a biological point of view, the apical one-third is a critical zone because it is the most difficult portion to clean and disinfect.2 As such, root-appropriate shaping is directed toward preparing a canal with a conservative body and deep shape.
Deep shape shortens the length of the lateral anatomy, increases the volume of an irrigant, and facilitates the exchange of irrigant into all aspects of the root canal system (Figure 2).3
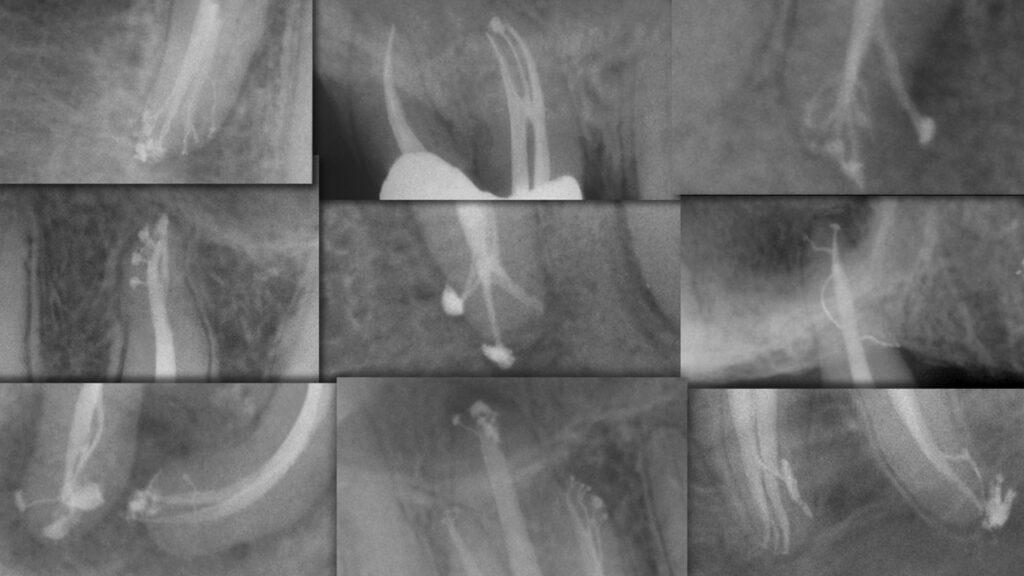
Figure 2. This collage of post-treatment images demonstrates the significance, rationale, and benefit of endodontic deep shape.
The absence of adequate shaping places an almost fanatical emphasis on utilizing controversial disinfection technologies and unproven obturation methods.
There are only a few 3D disinfection technologies in the marketplace that claim to not require canal preparation as traditionally advocated. The Lightwalker Er:YAG laser (Fotona) and GentleWave (Sonendo) are examples of technologies designed to clean more minimally instrumented canals prepared to a size 15/04 or 20/02.4 However, in the case of GentleWave, a recent scientific article reported an abundance of remaining micro-organisms in canals minimally prepared to a size 20/02 and “cleaned” with the GentleWave system.5
Further, do the frequent reports of unintended consequences following the disinfection cycle and the high costs associated with using this technology fulfill the value proposition?
This article will describe the sonic advantage, focus on a system-integrated technology that may be utilized for 3D cleaning in root-appropriate shapes, and provide the clinical protocol.
THREE-DIMENSIONAL DISINFECTION IN APPROPRIATELY SHAPED CANALS
Experience suggests most dentists performing endodontics strongly desire a seamless workflow that is directed toward root-appropriate shaping that enables the utilization of 3D cleaning and filling methods that are evidence-based, easy to use, and readily affordable.
The EndoActivator (Dentsply Sirona) is such a technology; has been utilized widely in the marketplace since 2007; and has been featured in more than 50 scientific, peer-reviewed published papers. That’s the good news; the better news is the new SmartLite Pro (SLP) EndoActivator builds on that cleaning and disinfection technology and can advantageously be utilized to perform other procedures as well (Figure 3).

Figure 3. This image compares the original EndoActivator to the SmartLite Pro (SLP) EndoActivator (Dentsply Sirona), which provides twice the frequency and a multi-directional tip movement.
Let’s now examine the sonic advantage for 3D cleaning.
THE SONIC ADVANTAGE
Sonic technology produces frequencies in the range of 10,000 to 20,000 cpm and, importantly, has been shown to be superior to ultrasonic technology when utilized for 3D disinfection.6 It is important to know the mechanical variables that most influence 3D cleaning. The streaming velocity of an irrigant prognosticates cleaning potential, and the variables include frequency (f); amplitude (a); and the radius (r), or the cross-sectional dimensions, of a tip. This concept is best appreciated by the mathematical formula: streaming velocity (v) = 2πfa2/r.
This formula identifies the variables that most influence the hydrodynamic phenomenon, which results when a vibrating tip generates fluid activation and intracanal waves. Random waves fracture, resulting in the formation of bubbles that oscillate within any given reagent. These bubbles are unstable due to heat and pressure, expand, and then collapse and implode. Each implosion generates up to 30,000 shockwaves that serve to powerfully penetrate, break up potential biofilms, and wipe surfaces clean.7
Frequency and Amplitude
The frequency is the interval of time it takes a vibrating tip to move through one back-and-forth displacement cycle. Upon activation, a sinusoidal wave of energy travels over the length of the insert tip. For 3D disinfection, clinicians have traditionally used ultrasonic technology to drive metal insert tips at a frequency of approximately 40,000 cps. However, an ultra-high frequency requires a super-low amplitude to mitigate tip breakage. Ultrasonically driven metal insert tips contribute to many iatrogenic events in both straighter and more curved canals.
Amplitude is the maximum back-and-forth displacement value of a vibrating tip. Advantageously, sonic technology produces a super-high tip amplitude that is about 50 times greater than ultrasonic technology (Figure 4).
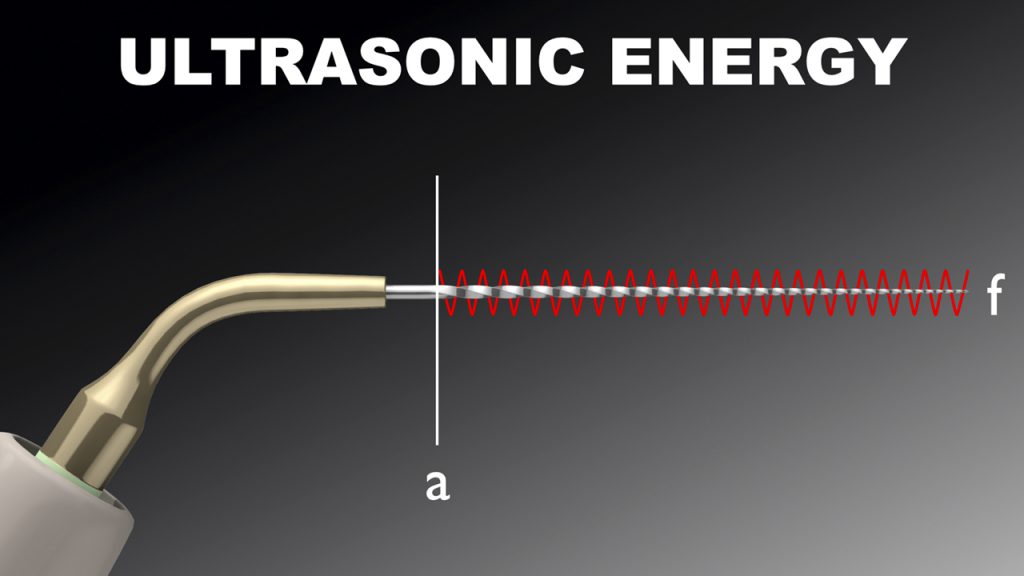
Figure 4a. Ultrasonic energy delivers an ultra-high frequency with an ultra-low amplitude to drive a metal insert tip.
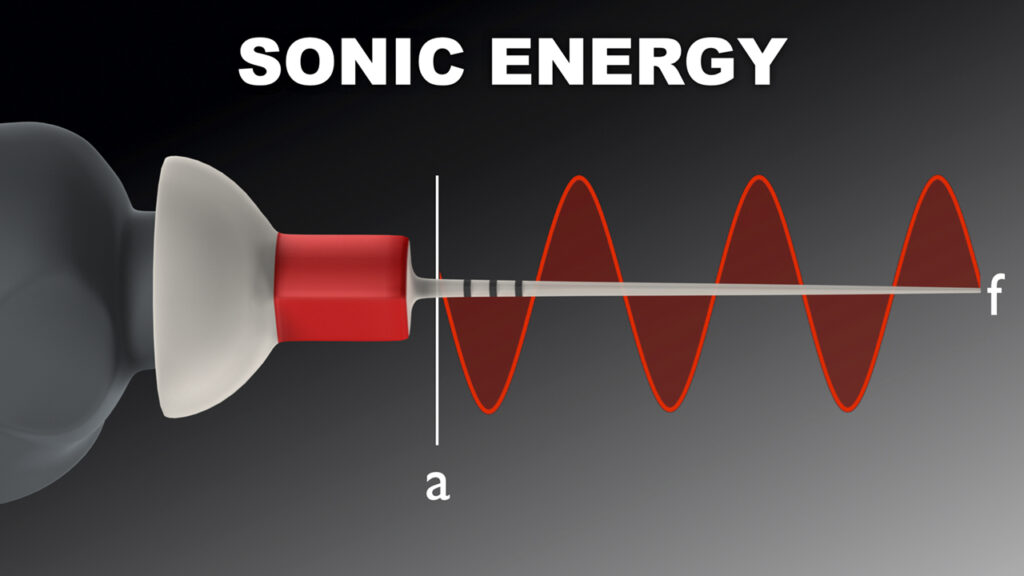
Figure 4b. Sonic energy delivers an ultra-low frequency with an ultra-high amplitude to safely drive a polymer snap-on tip.
In accordance with the above formula, a high amplitude value exponentially impacts streaming velocity. Fortuitously, sonic energy drives highly flexible, strong, polymer tips that will not break when utilized with a large amplitude value. Amplitude is the single most important factor to maximize 3D disinfection.4,6,8,9
Noncutting
Sonic technology drives highly flexible, noncutting, polymer tips that absolutely maintain the anatomical integrity of the final preparation.8,9 On the contrary, all ultrasonically driven instruments are manufactured from metal alloys and are either active and have cutting edges or are nonactive in that their cutting edges have been reduced or eliminated.
Regardless, any ultrasonically activated metal tip that contacts dentin will cut dentin and generate its own smear layer. Vibrating any metal tip, even pre-curved, around a canal curvature invites internal ledges, apical transportations, lateral perforations, or broken instruments (Figure 5).10
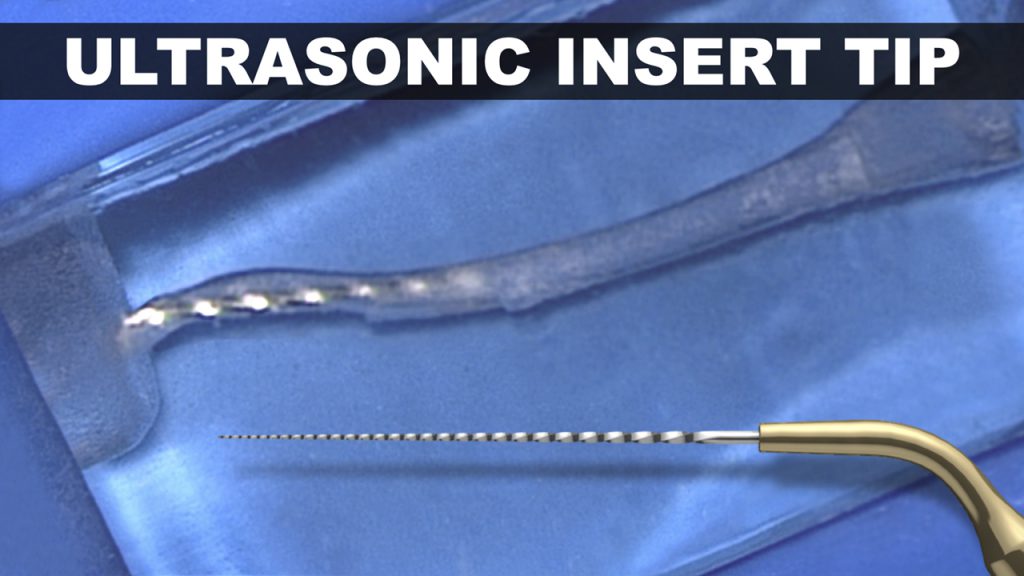
Figure 5a. In this plastic S-block, note the danger of using a metal insert tip around root curvatures, which has resulted in both internal and external transportations.
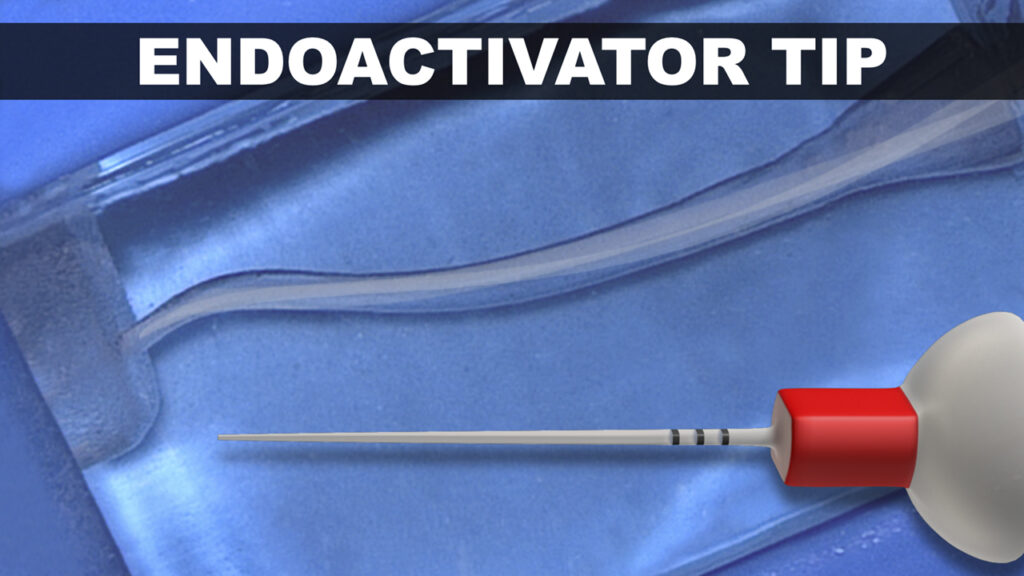
Figure 5b. In this plastic S-block, note the advantages of a noncutting, flexible polymer tip for 3D disinfection.
Continuous Movement
It should be understood that, even in well-shaped canals, any vibrating tip will certainly contact dentin. Research has shown that when a sonically activated polymer tip is constrained against a dentinal wall, the entire length of the tip advantageously continues to display a large displacement amplitude.11
To validate this sonic phenomenon, purposefully constrain a vibrating sonic tip at any level and note that, apical to the constrainment, the tip will continue to vigorously move!
On the contrary, constrain an activated ultrasonic insert tip and note the tip movement will be sharply reduced or the tip will not move at all; this reduces the exchange of any irrigant and potential to clean.
EVOLUTION OF THE TECHNOLOGY
Since 2007, the EndoActivator system has been available as a cordless, battery-operated handpiece that utilizes sonic energy to drive snap-on polymer tips.
Now, as a result of technological advancements, this system has evolved into a newly designed and considerably improved EndoActivator that is now a component part of the SmartLite Pro (SLP) multi-functional platform. Let’s look at this new system.
Platform System
The SLP platform is a unique, modular design, and multi-functional platform composed of a cordless, pen-style handpiece and 3 interchangeable, quick-connect attachments. Of the 3 attachments, one provides a curing light, a second is for transillumination, and the third is a sonically powered SLP EndoActivator attachment.
This latter attachment is designed to drive new and improved snap-on polymer tips, which, in turn, serve to activate and exchange an irrigant (Figure 6).

Figure 6. The SLP is a battery-powered system that provides 3 attachments. The EndoActivator attachment drives highly flexible, noncutting polymer tips.
Lastly, the SLP platform provides 2 latest-generation lithium iron phosphate batteries to power the handpiece and any given attachment. The platform is designed to organize and simplify the clinical workflow, but the clinician has a choice and can acquire any attachment, as desired. Let’s look at the SLP EndoActivator’s mode of action and the new polymer tips.
Handpiece and Disinfection Attachment
The SLP handpiece and EndoActivator attachment (SLP EndoActivator) operates at a frequency of 18,000 cpm. Importantly, the SLP EndoActivator drives newly designed polymer tips in a novel, random elliptical motion, whereas the first-generation EndoActivator utilized a back-and-forth linear motion.
Research shows that, beyond producing the hydrodynamic phenomenon, this multi-directional motion mechanically hits more internal canal walls, synergistically increasing the cleaning action (Figure 7). For infection control, a custom protective barrier sleeve easily slides over the entire SLP EndoActivator.
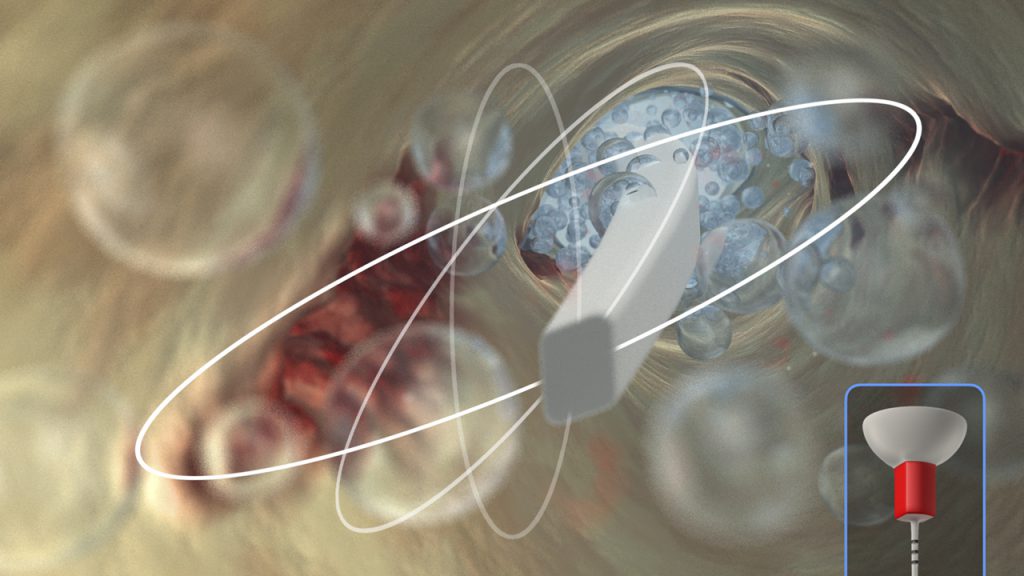
Figure 7. The SLP EndoActivator advantageously drives newly designed polymer tips in a novel, random, and elliptical motion.
After the disinfection cycle, the SLP handpiece and EndoActivator attachment are simply wiped clean with a mild disinfectant.
Tips and Selection
The SLP EndoActivator tips have a new paddle-like, parallelogram-shaped cross-sectional design to improve fluid exchange and cleaning potential (Figure 8).
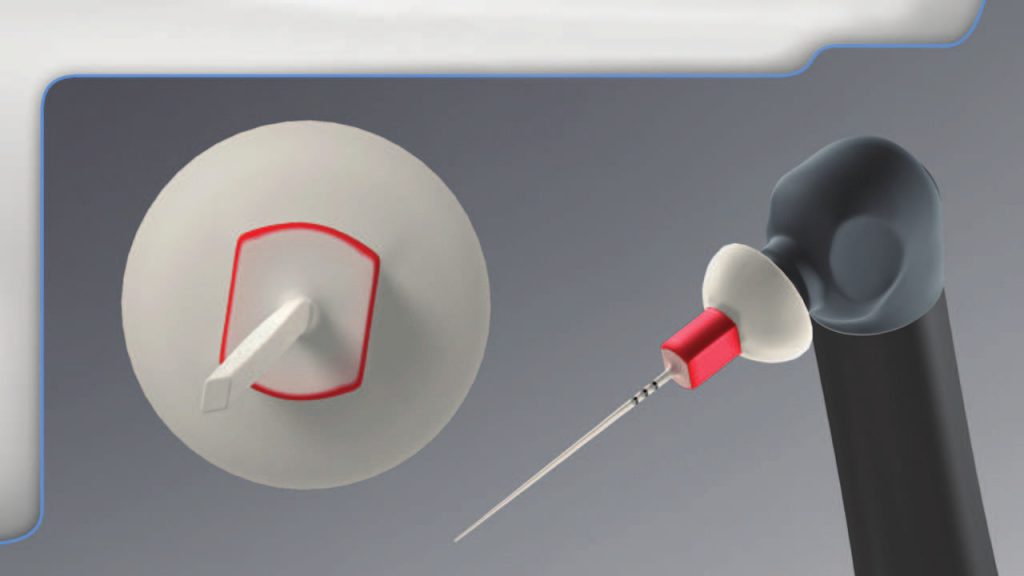
Figure 8. The newly designed polymer tips provide a paddle-shaped cross section to improve the exchange of an irrigant into the uninstrumentable anatomy
These new-generation tips are color-coded yellow and red to approximately correspond to file sizes 20/02 and 25/04, respectively.
The tips are made from a noncutting, medical-grade polymer; are strong and flexible; and have orientational depth gauge rings positioned at 18, 19, and 20 mm. The yellow tip is available in a 22-mm length, while the red tip is available in 22-mm and 28-mm lengths. The EndoActivator tips are single-use devices that should not be autoclaved or placed in any sterilizing solution, as this reduces elasticity, which in turn decreases tip amplitude and performance.
The protocol is to select a tip that fits loosely to within 2 mm of working length. Tip placement is easy in root-appropriate shapes, such as those cut by the ProTaper Ultimate F1 (20/07) or F2 (25/08) regressively tapered Finishing files. Moving an activated polymer tip in short 2- to 3-mm vertical strokes during the disinfection cycle synergistically produces a powerful hydrodynamic phenomenon (Figure 9).
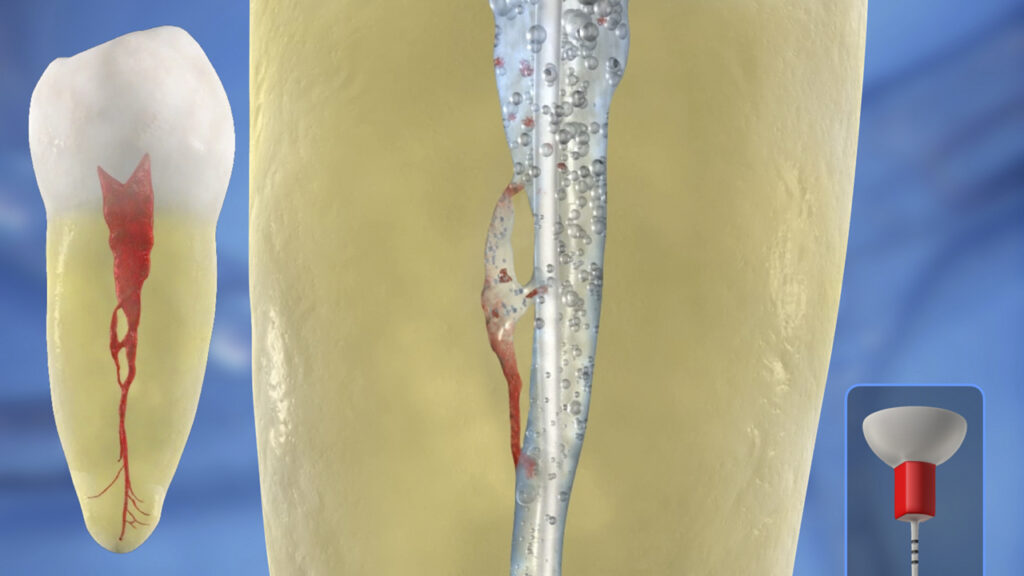
Figure 9a. This video animation image demonstrates the importance of dynamic irrigation to exchange irrigant into the uninstrumentable anatomy.
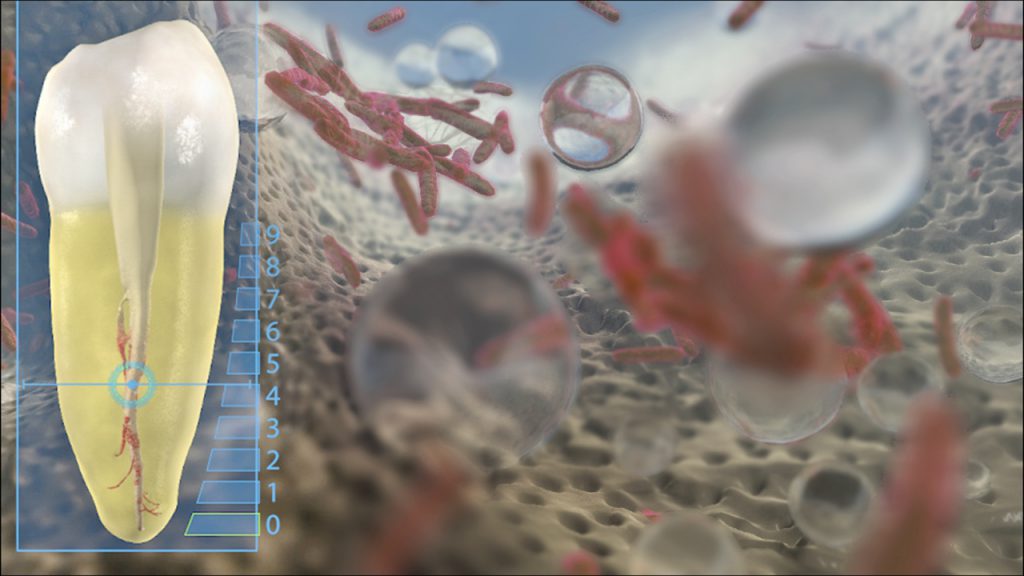
Figure 9b. This video animation image reveals how bubbles expand, collapse, and implode to generate shockwaves, which serve to wipe surfaces clean.
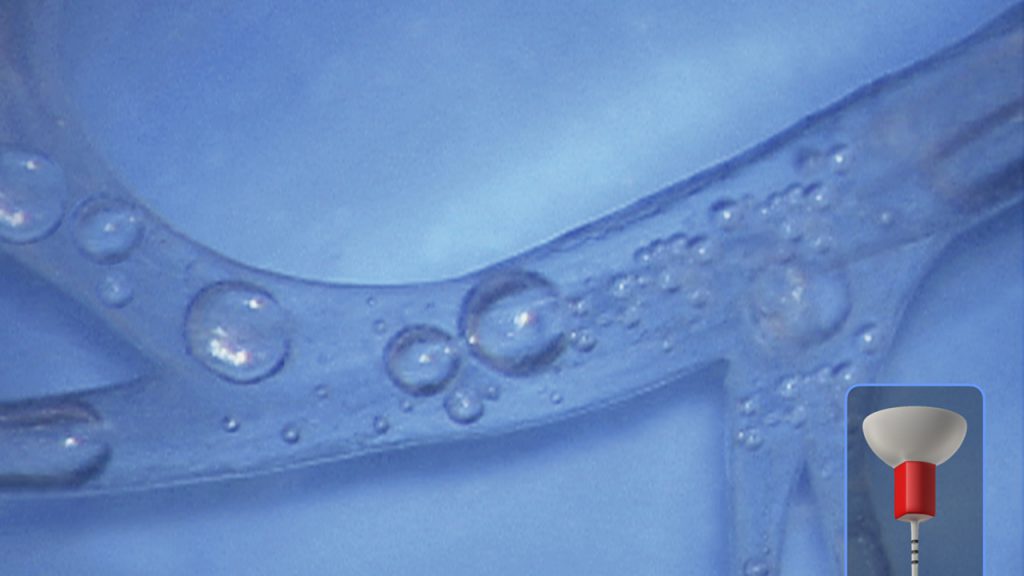
Figure 9c. Note that dynamic irrigation exchanges irrigant into all aspects of this simulated root canal system.
Scientific evidence supports that this specific technology debrides tissue remnants, eliminates the smear layer, and disrupts biofilms (Figure 10).6,8,9
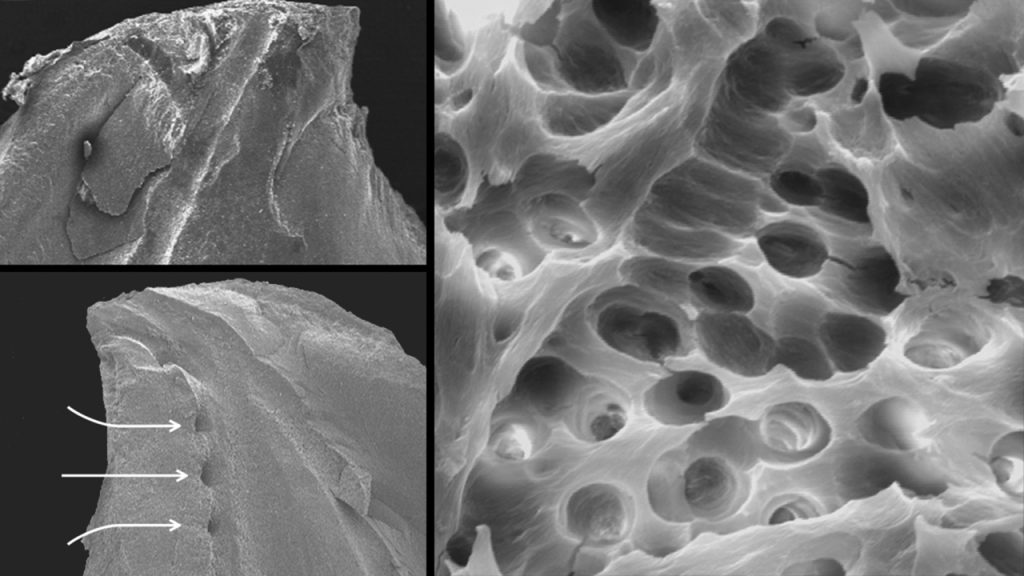
Figure 10. Three SEM images provide evidence that the EndoActivator system can clean root canal systems (Courtesy of Dr. Grégory Caron, Paris).
CLINICAL PROTOCOL
Following the shaping procedure, the root canal space is flushed with a 6% solution of NaOCl, then suctioned and removed. The pulp chamber is then flooded with a 17% solution of EDTA, as this reagent removes the smear layer, which serves to open up the uninstrumentable anatomy for more effective lateral exchange and deep cleaning.
The selected tip is placed over the protective barrier sleeve and utilized in a crown-down manner to agitate this solution of EDTA for 60 seconds per canal.
“Crown-down” means agitating this reagent for 5 seconds starting in the coronal one-third, then another 5 seconds in the middle one-third, and finally moving deeper to within 2 mm of working length to finish the 60-second disinfection cycle (Figure 11).
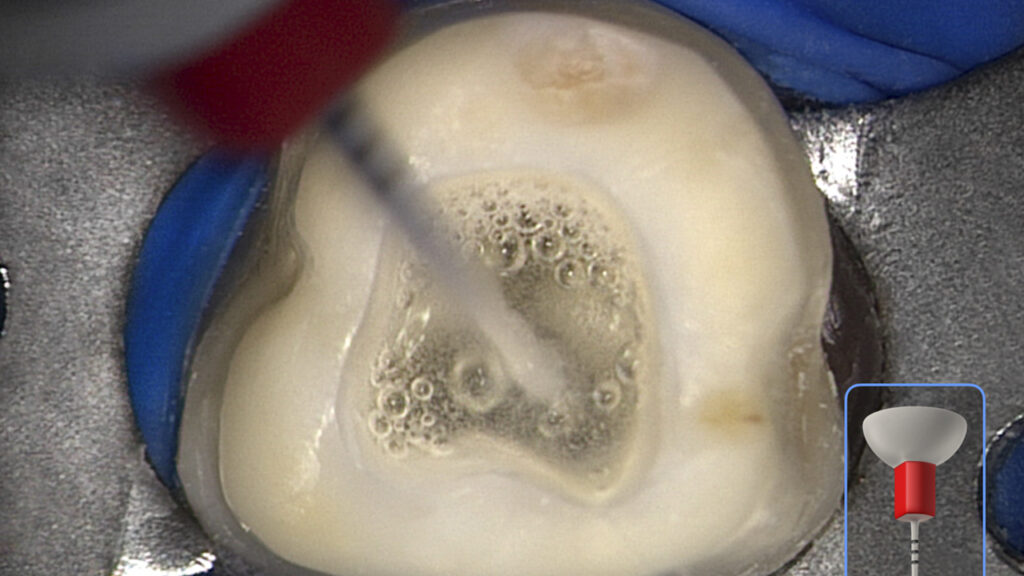
Figure 11. This clinical image shows the SLP EndoActivator in use. Note the powerful activation of fluid and appreciate the suborifice potential for 3D cleaning.
This crown-down method creates a powerful vortex where, upon activation, the fluid flow around the axis of the activated tip has microscale shape characteristics similar to a macroscale tornado. This disinfection process should be repeated for each canal or until the fluid in the pulp chamber is clinically observed to be visually clear.
After agitating 17% EDTA for 1 minute per canal, remove this reagent by vacuum. The root canal space is voluminously flushed with a 6% solution of NaOCl, and then this solution is activated for 30 seconds per canal to encourage deep penetration, circulation, and 3D cleaning (Figure 12).

Figure 12. (Left) An SEM image demonstrates that both the instrumented and uninstrumented surfaces are free of organic material. Note the deep lateral cleaning into the dentinal tubules. (Right) A stained axial cross section demonstrates the depth of exchange of silver ions deep into the dentinal tubules after using the SLP EndoActivator (Courtesy of Dr. Roberta Pileggi, Gainesville, Fla).
When the clinical procedure has been completed, the single-use activator tip and barrier sleeve are discarded.
ADJUNCTIVE USES
We have discussed the SLP EndoActivator in terms of 3D disinfection, but this technology is frequently used to perform other clinical procedures as well. For example, the SLP EndoActivator may be utilized to adapt and remove calcium hydroxide, or to move and adapt MTA into a wide-open canal or root defect, or to agitate an intracanal solvent.12
The latter serves to remove residual obturation remnants more effectively in the retreatment situation. In these clinical situations, the on/off switch on the SLP handpiece is quickly pressed twice to lower the frequency from 18,000 cpm to 3,000 cpm to best perform these adjunctive procedures.
To learn more about the various clinical uses of the SLP EndoActivator, go to endoruddle.com.
CLOSING COMMENTS
The goal of global endodontics should be to provide the greatest number of international patients with a 3D disinfection method that is safe, effective, and readily affordable. Importantly, the 3D disinfection method selected should be easy to use and fit seamlessly into a workflow that takes into consideration each pillar of the endodontic triad and its role in endodontic success. If you have the desire to treat root canal systems and are looking for predictability, possibility, and practicality, look no further than the SmartLite Pro EndoActivator.
REFERENCES
1. Schilder H. Cleaning and shaping the root canal. Dent Clin North Am. 1974;18(2):269–96.
2. Zehnder M. Root canal irrigants. J Endod. 2006;32(5):389–98. doi:10.1016/j.joen.2005.09.014
3. Machtou P, West JD, Ruddle CJ. Deep shape in endodontics: significance, rationale, and benefit. Dent Today. 2022;41(1):4-77.
4. Ruddle CJ. Endodontic Triad for Success: The Role of Minimally Invasive Technology. Dent Today. 2015;34(5):76, 78-80.
5. Ordinola-Zapata R, Mansour D, Saavedra F, et al. In vitro efficacy of a non-instrumentation technique to remove intracanal multispecies biofilm. Int Endod J. 2022. doi:10.1111/iej.13706
6. Kanter V, Weldon E, Nair U, et al. A quantitative and qualitative analysis of ultrasonic versus sonic endodontic systems on canal cleanliness and obturation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112(6):809–13. doi:10.1016/j.tripleo.2011.06.002
7. Gutarts R, Nusstein J, Reader A, et al. In vivo debridement efficacy of ultrasonic irrigation following hand-rotary instrumentation in human mandibular molars. J Endod. 2005;31(3):166–70. doi:10.1097/01.don.0000137651.01496.48
8. Ruddle CJ. Endodontic disinfection: tsunami irrigation. Endodontic Practice. 2008;11(1):7-15.
9. Caron G, Nham K, Bronnec F, et al. Effectiveness of different final irrigant activation protocols on smear layer removal in curved canals. J Endod. 2010;36(8):1361–6. doi:10.1016/j.joen.2010.03.037
10. Ruddle CJ. Chapter 25: Nonsurgical endodontic retreatment. In: Cohen S, Burns RC, eds. Pathways of the Pulp. Mosby; 2002:875-929.
11. Walmsley AD, Lumley PJ, Laird WR. Oscillatory pattern of sonically powered endodontic files. Int Endod J. 1989;22(3):125–32. doi:10.1111/j.1365-2591.1989.tb00910.x
12. Ruddle CJ. Endodontic advancements: game-changing technologies. Dent Today. 2009;28(11):82, 84.
ABOUT THE AUTHOR
Dr. Ruddle is founder and director of Advanced Endodontics, an international educational source, in Santa Barbara, Calif.
Further, he is the creator of The Ruddle Show


