INTRODUCTION
It has been well-substantiated that extractions (without supplemental guided bone regeneration [GBR], or “unassisted”) can lead to loss of horizontal and vertical ridge dimensions. Numerous studies have concluded that the use of GBR techniques with barrier membranes minimizes the loss of alveolar bone height and width. This is of special concern in the aesthetic zone, in areas of unfavorable pontic anatomy, and in future implant sites. It is imperative to evaluate socket anatomy and compromised bony housing before treating socket defects. The benefits of laser-assisted socket management in the treatment of these defects will be addressed, as will a step-by-step protocol. These case reports present a novel technique for laser-assisted socket grafting (LASG) without the use of barrier membranes.
Tooth eruption studies have shown that the alveolar process is dependent on the presence of a tooth. Surrounding bone volume is related to the shape, size, inclination, and eruption of the tooth, along with genetic and developmental factors. The tooth is housed by bundle bone, which is penetrated by the periodontal ligament fibers.1
In a systematic review, Van der Weijden et al2 found a mean clinical loss of 3.87 mm in width and 1.53 mm in height (radiographically) with unassisted socket healing. Similarly, in a more recent review, Tan et al3 found a mean horizontal loss of 3.79 mm and a vertical loss of 1.24 mm with unassisted socket healing. When socket preservation therapies were used, a gain in width of 1.83 mm and a gain in height of 1.47 mm were found in test vs control groups.4 It was concluded that horizontal resorption is more pronounced than vertical loss and dimensional changes of the alveolar ridge can be limited by GBR techniques.5
Nowadays, the most important reason for socket grafting is to preserve the form of the ridge for a restoratively driven implant.6 The survival rate of fixtures placed in grafted sites is similar to those placed into native bone.7 The principles of GBR, which include maintenance of space, angiogenesis within the grafted material, prevention of epithelial down-growth, and stabilization of the blood clot during healing, are crucial to the success of socket grafting. This is especially important in deficient sockets, such as Funato’s Class III and IV or Elian’s Type III sockets.8,9
The Nd:YAG laser is a useful instrument for assisting with multiple aspects of extraction socket management. When the Nd:YAG laser tip is applied to the sulcular collar around periodontally unhealthy teeth, it ablates the ulcerated epithelium, leaving healthy connective tissue intact.10 The laser energy can function similarly when applied to residual unhealthy tissue in an extraction socket. This specific laser wavelength eliminates periodontal pathogens in the periodontal pockets of the tooth slated for extraction.11,12 Additionally, Nd:YAG lasers can potentially convert granulomatous tissue (tissues that have inflammatory components and pathogens) into granulation tissue. Granulation tissue has cellular components, possibly stem cells,13 and vascularity important for the regeneration process. The Nd:YAG can transform blood into a “thermogenic clot.” This acts as a membrane protecting the site from epithelial down-growth. Lasers have been shown to help in repair14-16 and regeneration17-19 of bony defects as well as in providing biostimulation and pain reduction.20
This article will present clinical case reports utilizing an LASG technique, dependent on the anatomy of the socket, in preparation for implant placement.
MATERIALS AND METHODS
1. After confirming that the tooth was required to be extracted (Figure 1), an “atraumatic” extraction protocol was followed to minimize trauma to the adjacent tissue and bone. Following administration of local anesthetic, a 360° intrasulcular incision was made. Periotomes and appropriate forceps were then used to deliver the tooth.
2. The sulcular ulcerated tissue was ablated with an Nd:YAG laser (Millennium Dental Technologies) placed alongside the socket wall to the accessible depth of the base of the pocket (Figure 2).
3. The socket was gently debrided. The laser was then used to remove bacterial pathogens from the granulomatous tissue. Laser ablation alters this pathological tissue (Figure 3). The residual remaining granulation tissue has cellular components, vascularity, and possible growth factors to aid with socket regeneration.
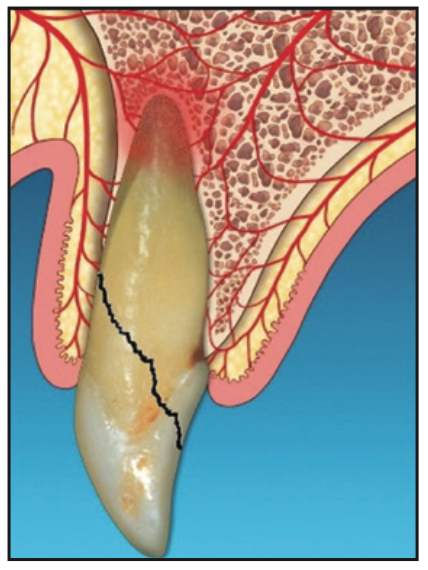
Figure 1. Diagnosis of non-restorable tooth.
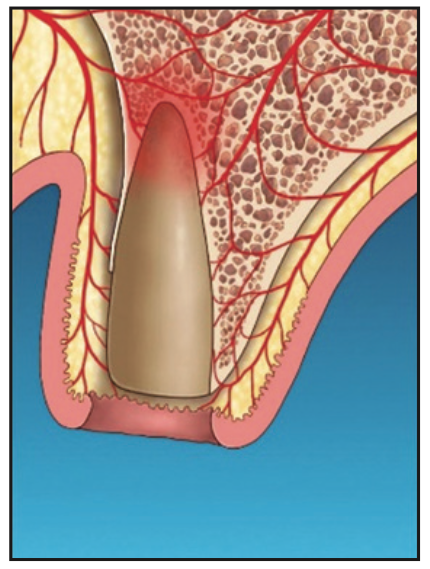
Figure 2. Laser ablation to remove ulcerated epithelium at the internal coronal marginal area.
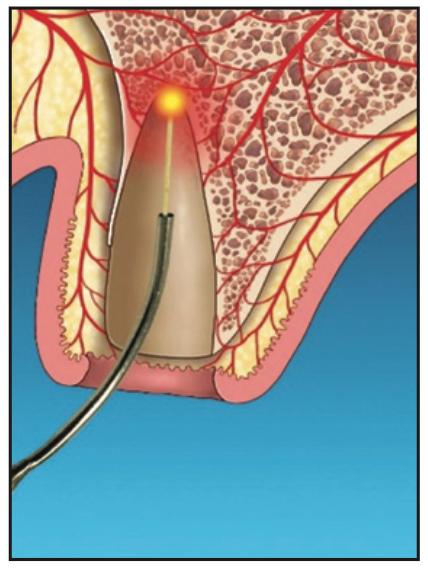
Figure 3. Laser detoxification, leaving cellular components, vascularity, and potential growth factors for regeneration.
4. Following socket laser ablation and detoxification, the piezo tip was used to penetrate the surrounding bone. This decortication was intended to further release potential healing growth factors and stem cells into the socket (Figure 4).
5. After allowing an initial clot to form, the Nd:YAG laser was set on a hemostasis mode. The socket was lased for hemostasis (Figure 5), and afterward, a firm gelatinous clot was formed.
6. This clot would act as a barrier membrane to contain graft materials (such as particulate allograft, xenograft, or a calcium phosphate plug and/or block) that conforms to the residual anatomy of the socket and prevent epithelial migration into the socket (Figure 5).
7. If socket walls are missing, a semi-solid collagen plug incorporated with graft material (eg, an OsteoGen Bone Grafting Plug [IMPLADENT LTD]) or an allogenic block can be shaped to conform to the socket. The extracted root can be used as a template to shape the block. The material is placed into the clot approaching the accessible base of the socket (Figure 6).
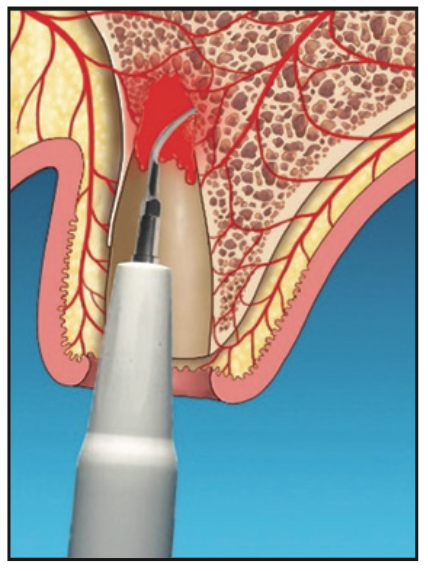
Figure 4. Decortication/bone modification to potentially release growth factors and stem cells.
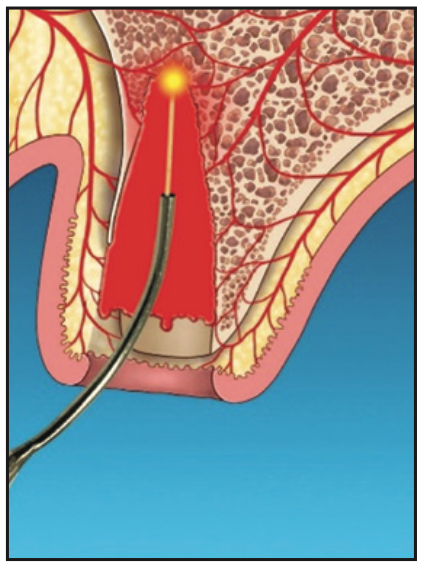
Figure 5. Laser hemostasis to form a thermogenic clot that acts as a barrier to epithelial penetration.
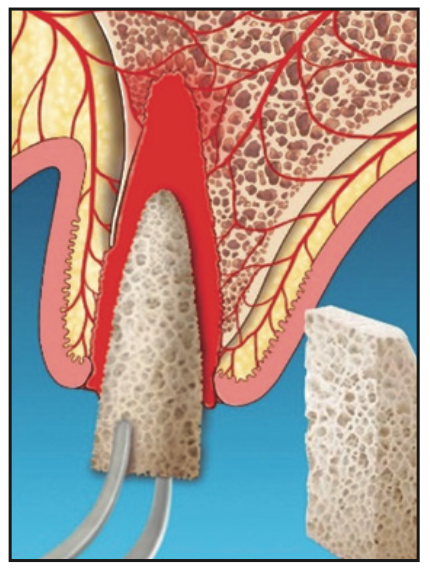
Figure 6. Depending on space maintenance requirements, either a particulate graft, plug, or block is placed into the clot.
8. If the clot has been disrupted after graft material placement, the site is lased again with the Nd:YAG in hemostasis mode using 450 to 650 pulse duration, depending on the amount of bleeding (Figure 7). Postoperative instructions are similar to those for routine extractions and GBR protocols. The patient was advised not to disrupt the clot. Gauze placement and biting are not recommended. The patient was asked to refrain from hot liquids, rinsing, exercise, and disruption of the clot for 48 hours.
9. The site was monitored clinically and radiographically for complete soft-tissue and hard-tissue maturation (Figure 8).
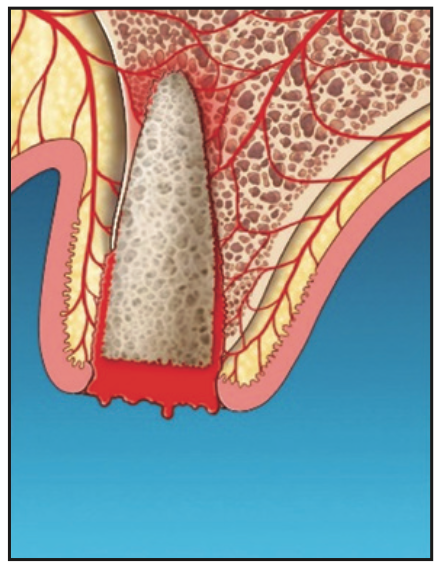
Figure 7. Determination of laser hemostasis and additional laser hemostasis, if required.
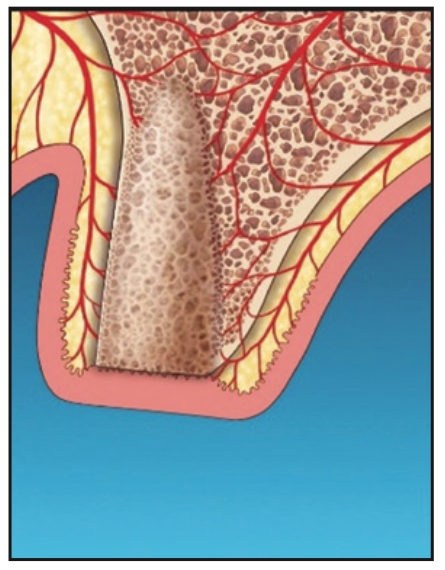
Figure 8. Adequate osseous fill following laser-assisted socket grafting.
CASE REPORTS
Case 1
A 17-year-old male was referred to the Department of Periodontology at the Rutgers School of Dental Medicine (RSDM). The patient’s medical history revealed no systemic contraindications for dental treatment. Clinical and radiographic examination revealed external root resorption with a history of trauma on the maxillary right central incisor (Figures 9 and 10). Using a PerioLase MVP-7 Laser (Millennium Dental Technologies), a sulcular incision was made in 360° in ablation mode. The tooth was extracted using periotomes and luxators (PDL-Evator Precise Tip Luxating Elevators [Salvin Dental Specialties]) without elevating a flap. Upon examination, a 6-mm buccal plate dehiscence was noted, and the decision was made to use a block allograft. The Nd:YAG laser (PerioLase MVP-7) was used on the hemostasis setting, 550 pulse duration, to administer 146 J to create a stable blood clot (Figure 11). A bone block (Puros Block Allograft [Zimmer Biomet]) was carved using the root as a template to mimic the shape of the extracted tooth (Figure 11). An additional 100 J were administered to complete hemostasis over the bone block. Post-op ibuprofen 400 mg, 20 tabs q6h prn pain, was prescribed. An Essix retainer with a pontic was fabricated as an interim prosthesis. The patient was instructed not to function on the temporary prosthesis during the healing phase. The patient was seen for a 2-week followup, and then every 4 weeks after that, for supragingival debridement and oral hygiene reinforcement. Dimensional stability and adequate clinical healing were noted at 3 months. A 3.3- × 12-mm implant (Bone Level Straight implant [Straumann]) was placed in good-quality bone with good stability and no complications (Figure 12). The final prosthesis was delivered 4 months following implant placement (Figure 13).
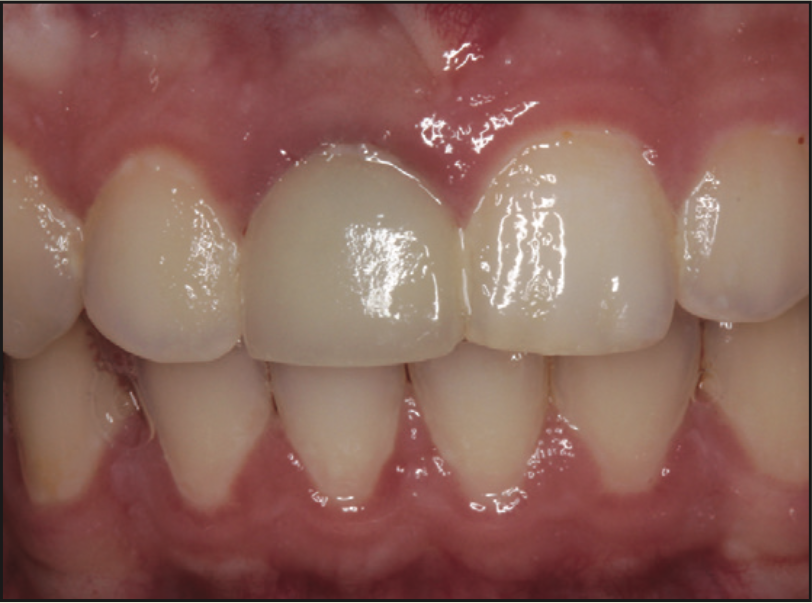
Figure 9. Preoperative view of the maxillary right central incisor.
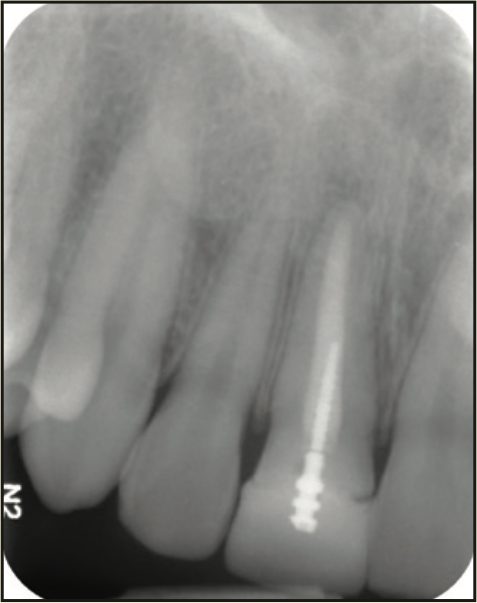
Figure 10. Pre-op radiograph.
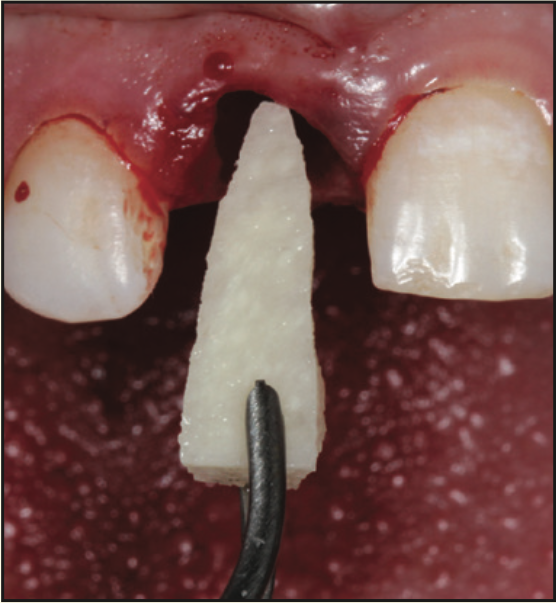
Figure 11. Placement of the carved allograft bone block into the socket following extraction and laser hemostasis.
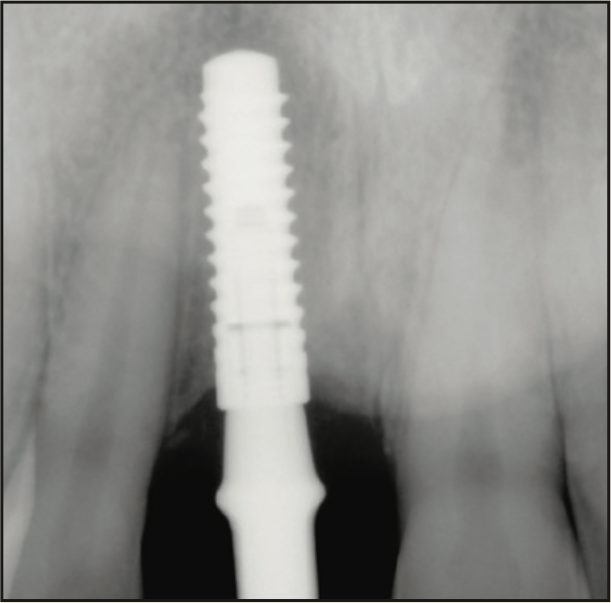
Figure 12. Radiograph of the implant 4 months after placement.
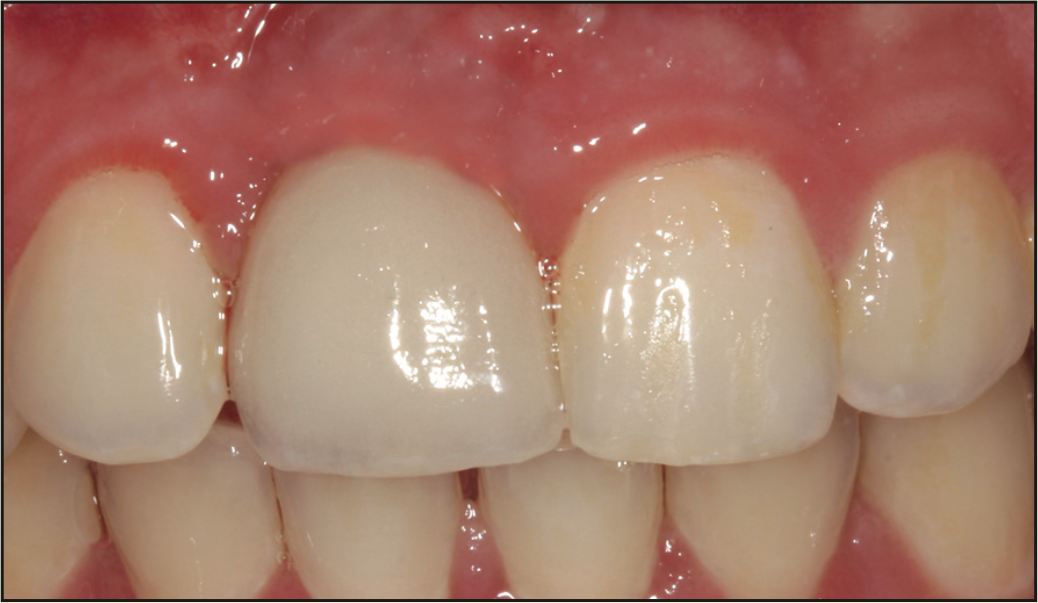
Figure 13. Final screw-retained crown.
Case 2
A 65-year-old male presented with remaining root tips of the mandibular right first molar (Figure 14). After an explanation of treatment options, the patient elected to have the tooth extracted and replaced with an implant-retained prosthesis. The remaining roots were extracted using elevators (Salvin Dental Specialties) without elevating a flap. The PerioLase MVP-7 Nd:YAG laser was used to ablate the remaining sulcular epithelium. Hemostasis was achieved using 158 J. Immediately after, particulate allograft (Puros Block Allograft) was placed into the socket. An additional 102 J were administered over the bone allograft because hemostasis was disrupted. No sutures or membrane was placed. Post-op medications of ibuprofen 400 mg, 20 tabs q6h prn pain, and Azithromycin 500 mg, 10 tabs QD, were prescribed. The healing phase was closely monitored and had no adverse events. After 3 months of healing, a trephine core biopsy was obtained. Histological analysis confirmed vital bone formation (Figure 15). A Full OSSEOTITE Tapered Certain Prevail 5/4- × 10-mm platform-switched implant was placed (3i [Zimmer Biomet]) (Figure 16). A screw-retained final restoration was placed after 4 months of healing (Figures 17 and 18).
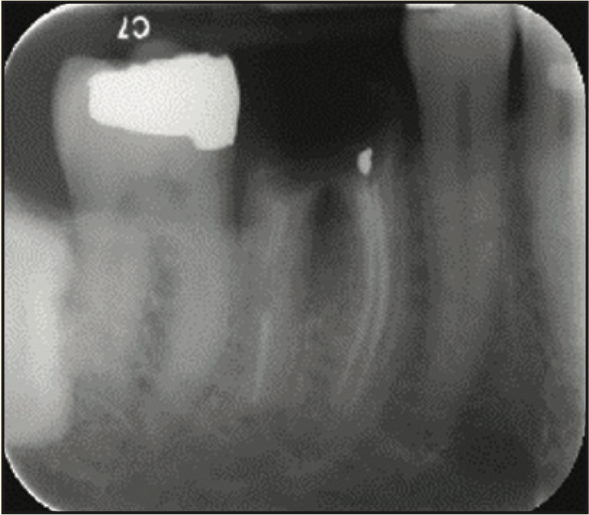
Figure 14. Pre-op radiograph of the remaining tooth structure.
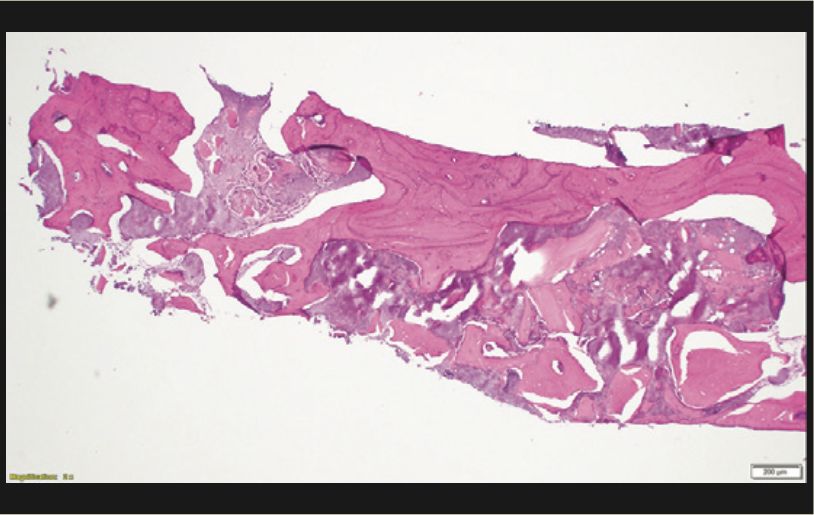
Figure 15. Histology showing vital new bone.
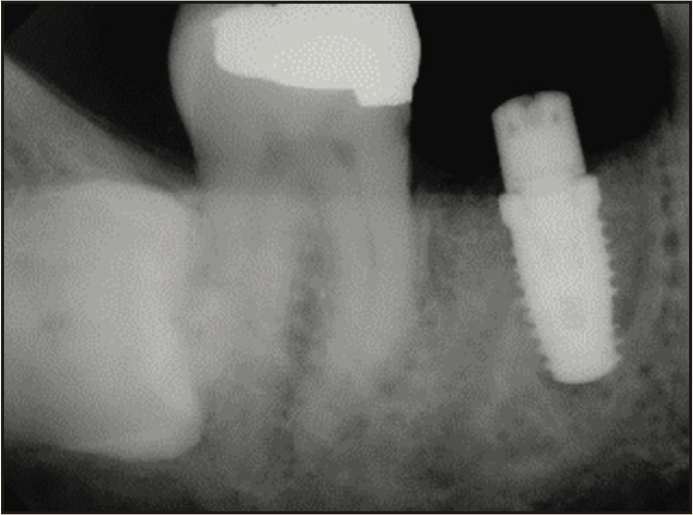
Figure 16. Radiograph of implant placement.
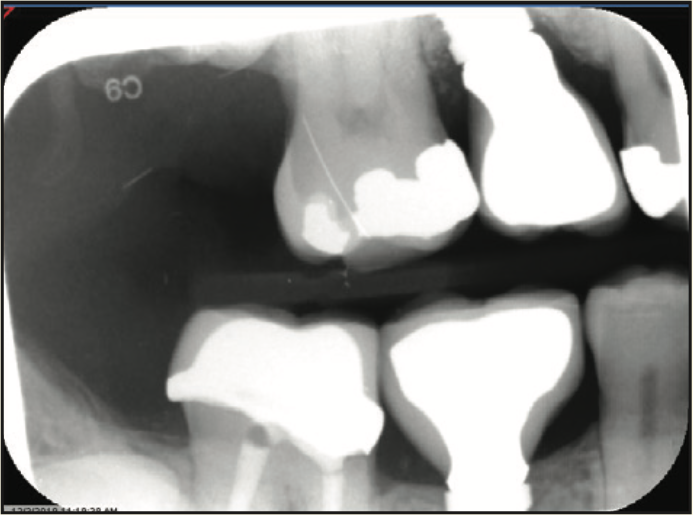
Figure 17. Radiograph of final crown placement.
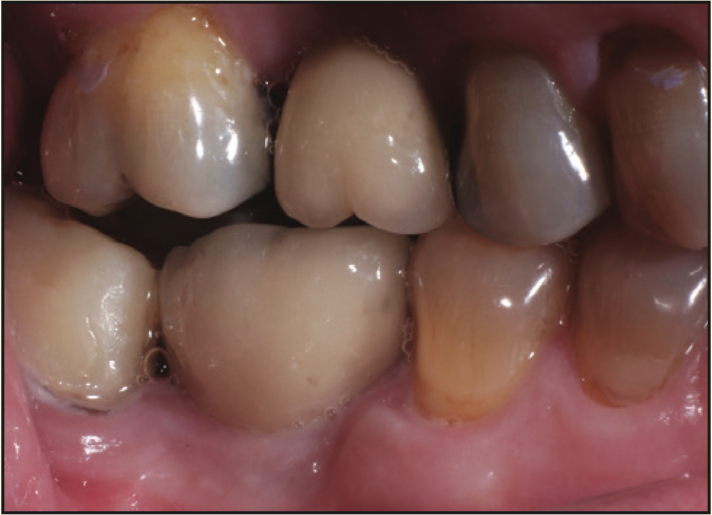
Figure 18. Buccal photograph of the screw-retained crown.
Case 3
A 73-year-old female presented with an apical lesion labial to her maxillary left central and lateral incisors. Suppuration and pain upon percussion were noted (Figure 19). Radiographic and CBCT exams revealed a through-and-through defect from the labial to palatal bone (Figure 20). The patient’s health history included total hip replacement and hyperparathyroidism. The patient was taking a bisphosphonate for osteoporosis. These factors contributed to the decision to not pursue an implant and to prepare the site for a pontic in a fixed bridge. An LASG technique (PerioLase MVP-7 Nd:YAG laser) provided a conservative approach, eliminating the need to elevate a flap and still meet the objective of bone repair and an aesthetic result. Two medium-sized absorbable collagen bullet-shaped plugs were used to ensure space maintenance of the defect. The plug was slightly larger than the roots to help maintain contact with the apical and interproximal bone.
The hemostatic clot that was formed after laser application provided a scaffold to secure the position of the plug and prevent its dislodgement from the socket. No sutures were needed (Figure 21). An 8-month periapical radiograph showed new bone fill at the site (Figure 22). The patient was restored with a new fixed prosthesis from Nos. 6 to 11 (Figure 23). An appropriate emergence profile was developed during the transitional healing phase, and no significant defect was noted.
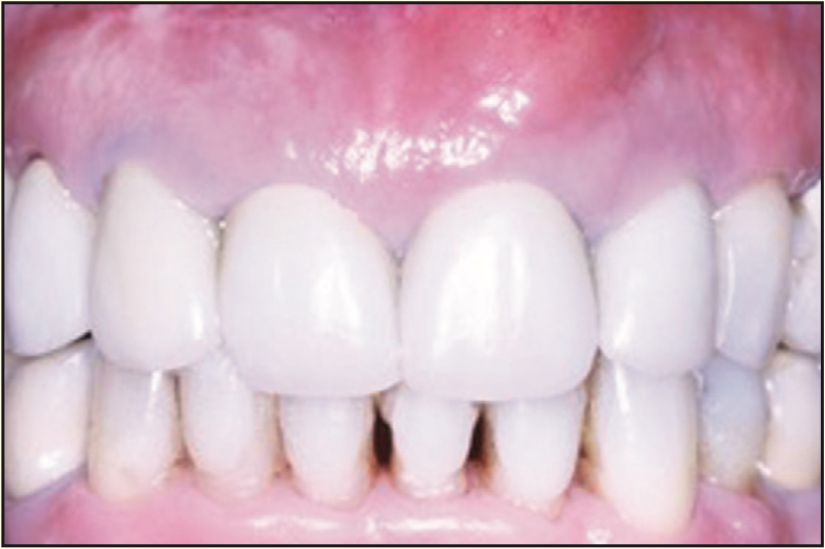
Figure 19. Pre-op photograph of the failing teeth with a large buccal swelling.
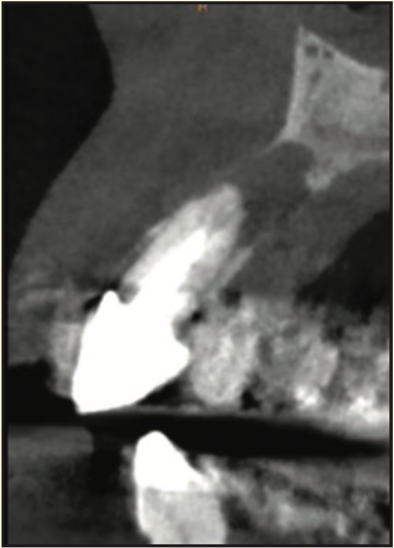
Figure 20. Pre-op CBCT radiograph of the infection around the maxillary left central incisor.
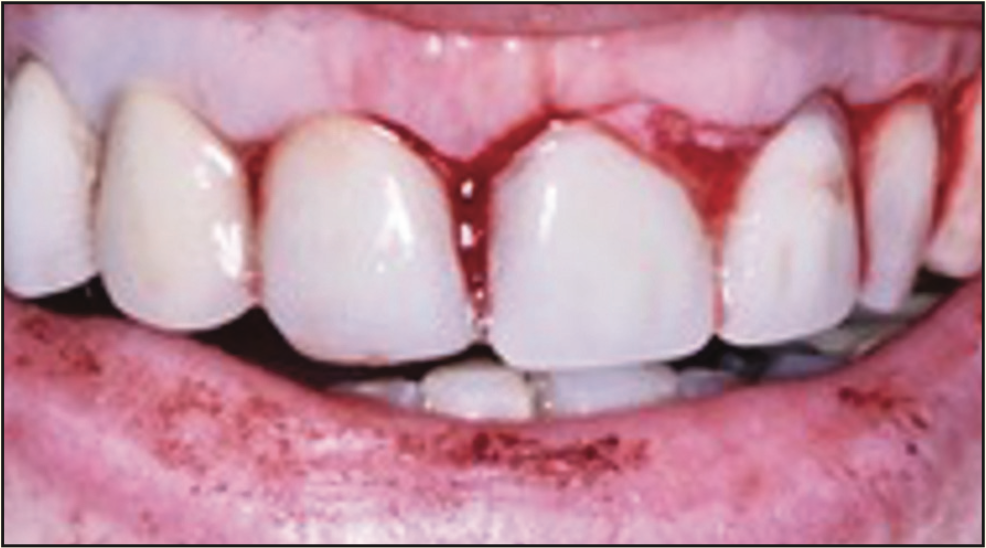
Figure 21. Immediate post-extraction photograph with provisional FPD in place. Post-extraction sockets had collagen sponges added following laser socket hemostasis.
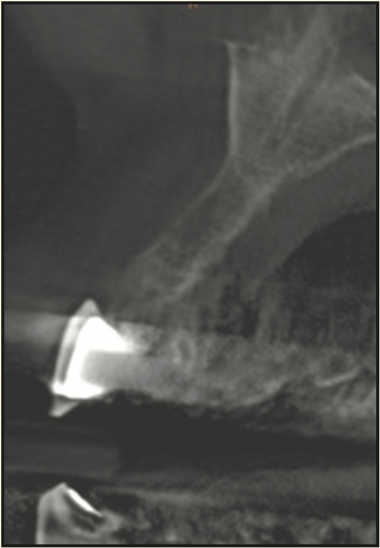
Figure 22. CBCT showing bone fill in previous area of severe bone resorption.
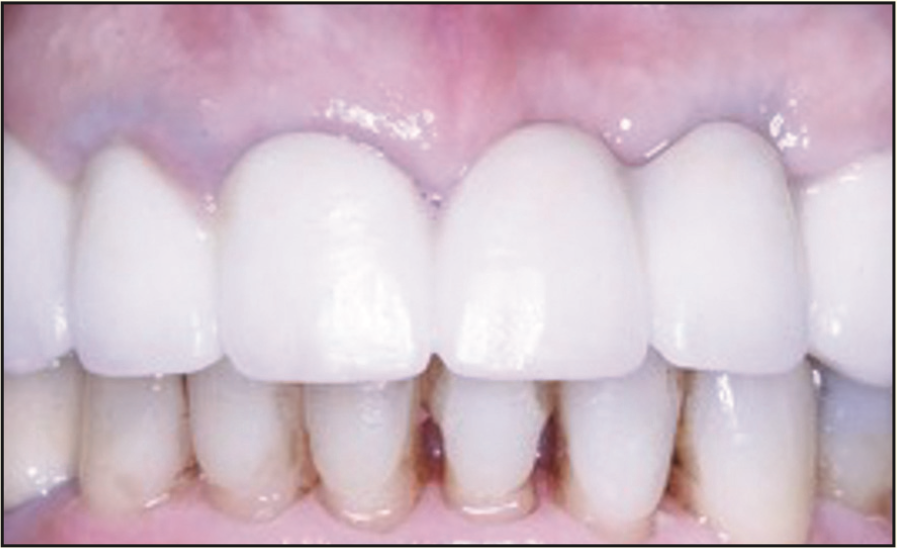
Figure 23. Seven months after crown insertion.
DISCUSSION
The laser socket grafting technique described was developed based on proven techniques and available literature. The initial step uses the laser to make an intrasulcular incision, which selectively ablates diseased epithelium but maintains healthy connective tissue. Gold and Vilardi10 evaluated human gingival tissue following the removal of pocket-lining epithelium with a pulsed Nd:YAG laser. Sections showed complete removal of epithelium (83%), with 17% having trace amounts of viable basal cell remnants at the coronal margin. The connective tissue showed no evidence of carbonization or necrosis.10 Ting et al21 performed a human histologic study using the Nd:YAG laser, demonstrating complete removal of the ulcerated epithelium without harming the underlying connective tissue. In patients with a thin phenotype, this is particularly important, especially compared to traditional incisions.
Following extraction, laser energy is used to reduce bacteria in the sockets. An in vitro study demonstrated that the pulsed Nd:YAG laser selectively destroyed pigmented pathogens.11 In 2014, a human study compared the antibacterial effects of laser periodontal therapy using an Nd:YAG laser vs ultrasonic root debridement. Eighty-five percent of patients treated with the laser-assisted new attachment procedure were culture-negative for all evaluated periodontal pathogens in immediate post-treatment samples. Patients treated within the ultrasonic group had 100% of culture samples remain positive for most subgingival bacterial pathogens recorded at baseline.12
If granulomatous tissue is present, the laser can detoxify the tissue. The remaining granulation tissue may provide vasculature and cellular components to aid in regeneration. Recently, Ronay et al13 removed granulation tissue from furcation and infrabony defects. Cells expressing embryonic stem cell markers and the tissue exhibiting osteogenic capacities were found when cultured in vitro.13 An in vivo animal model showed that periodontium-derived stem cells can be cultured and are capable of regenerating bone and collagen elements.22
The bone modification and decortication of the socket may release growth factor components. An in vitro study demonstrated that the Nd:YAG laser increased osteoblast activity and additionally accelerated mineral deposition. The authors speculated that the laser might activate BMP-2.23 In another in vitro study, the Nd:YAG laser induced gingival fibroblasts to proliferate and up-regulated the secretion of epidermal growth factor.24
A study comparing treatment of intrabony defects with and without intramarrow penetration (IP) demonstrated clinical and radiographic outcomes that were significantly improved with IP.25 Verdugo et al26 found that when performing GBR, cortical perforations may improve clinical and histological outcomes. The thick thermogenic clot formed with the Nd:YAG laser acts as a barrier membrane to exclude the epithelium and allow the necessary cellular components to form osteoid and, eventually, bone.
The determination to use a particulate graft, a semi-solid collagen plug, or an allogeneic block is based on principles of GBR. If additional space maintenance is needed, a plug or block is used. Reddy et al27 emphasized the importance of space maintenance in treating the atrophic socket. A benefit of the laser is that less post-op discomfort should be anticipated due to the photostimulation from the laser energy. Lasers have been shown to provide pain relief, improve wound healing, and stimulate collagen growth.28
CONCLUSION
These case reports present a novel socket grafting procedure based on GBR principles. Critical for success is the evaluation of the socket defect and whether particulate material, a plug, or a block graft is necessary for space maintenance. A sound thermogenic clot is an absolute requirement to act as a barrier membrane. With the improvement of CAD/CAM technology and grafting materials, the potential to create a plug or bone block based on root morphology will have the potential to aid in space maintenance for optimum bone regeneration. Additional research with LASG is recommended to improve and advance the technique of using the inherent blood clot and grafting material to enhance the patient outcome.
ACKNOWLEDGMENTS
We would like to thank Alireza Oryan for editing and formatting the manuscript prior to its submission.
REFERENCES
1. Marks SC Jr, Schroeder HE. Tooth eruption: theories and facts. Anat Rec. 1996;245(2):374–93. doi:10.1002/(SICI)1097-0185(199606)245:2<374::AID-AR18>3.0.CO;2-M
2. Van der Weijden F, Dell’Acqua F, Slot DE. Alveolar bone dimensional changes of post-extraction sockets in humans: a systematic review. J Clin Periodontol. 2009;36(12):1048–58. doi:10.1111/j.1600-051X.2009.01482.x
3. Tan WL, Wong TL, Wong MC, et al. A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin Oral Implants Res. 2012;23 Suppl 5:1-21. doi:10.1111/j.1600-0501.2011.02375.x.
4. Vignoletti F, Matesanz P, Rodrigo D, et al. Surgical protocols for ridge preservation after tooth extraction. A systematic review. Clin Oral Implants Res. 2012;23 Suppl 5:22-38. doi:10.1111/j.1600-0501.2011.02331.x
5. Horváth A, Mardas N, Mezzomo LA. Alveolar ridge preservation. A systematic review. Clin Oral Investig. 2013;17(2):341–63. doi:10.1007/s00784-012-0758-5
6. Tarnow DP, Eskow RN. Preservation of implant esthetics: soft tissue and restorative considerations. J Esthet Dent. 1996;8(1):12-9. doi:10.1111/j.1708-8240.1996.tb00904.x
7. Fiorellini JP, Nevins ML. Localized ridge augmentation/preservation. A systematic review. Ann Periodontol. 2003;8(1):321–7. doi:10.1902/annals.2003.8.1.321
8. Funato A, Salama MA, Ishikawa T, et al. Timing, positioning, and sequential staging in esthetic implant therapy: a four-dimensional perspective. Int J Periodontics Restorative Dent. 2007;27(4):313–23.
9. Elian N, Cho SC, Froum S, et al. A simplified socket classification and repair technique. Pract Proced Aesthet Dent. 2007;19(2):99-104.
10. Gold SI, Vilardi MA. Pulsed laser beam effects on gingiva. J Clin Periodontol. 1994;21(6):391-6. doi:10.1111/j.1600-051x.1994.tb00735.x
11. Harris DM, Yessik M. Therapeutic ratio quantifies laser antisepsis: ablation of Porphyromonas gingivalis with dental lasers. Lasers Surg Med. 2004;35(3):206–13. doi:10.1002/lsm.20086
12. McCawley TK, McCawley MN, Rams TE. LANAP Immediate Effects In Vivo on Human Chronic Periodontitis Microbiota. In J Dent Res; March 20, 2014. American Association for Dental Research 43rd Annual Meeting, Charlotte, NJ. 93 (Spec Issue A); Abstract 428.
13. Ronay V, Belibasakis GN, Attin T, et al. Expression of embryonic stem cell markers and osteogenic differentiation potential in cells derived from periodontal granulation tissue. Cell Biol Int. 2014;38(2):179–86. doi:10.1002/cbin.10190
14. Pinheiro AL, Limeira Júnior Fde A, Gerbi ME, et al. Effect of low level laser therapy on the repair of bone defects grafted with inorganic bovine bone. Braz Dent J. 2003;14(3):177-81. doi:10.1590/s0103-64402003000300007
15. Soares LG, Marques AM, Guarda MG, et al. Raman spectroscopic study of the repair of surgical bone defects grafted or not with biphasic synthetic micro-granular HA + β-calcium triphosphate irradiated or not with λ850 nm LED light. Lasers Med Sci. 2014;29(6):1927–36. doi:10.1007/s10103-014-1601-9
16. Monea A, Beresescu G, Boeriu S, et al. Bone healing after low-level laser application in extraction sockets grafted with allograft material and covered with a resorbable collagen dressing: a pilot histological evaluation. BMC Oral Health. 2015;15:134. doi:10.1186/s12903-015-0122-7
17. Nevins ML, Camelo M, Schupbach P, et al. Human clinical and histologic evaluation of laser-assisted new attachment procedure. Int J Periodontics Restorative Dent. 2012;32(5):497-507.
18. Nevins M, Kim SW, Camelo M, et al. A prospective 9-month human clinical evaluation of Laser-Assisted New Attachment Procedure (LANAP) therapy. Int J Periodontics Restorative Dent. 2014;34(1):21–7. doi:10.11607/prd.1848
19. Yukna RA, Carr RL, Evans GH. Histologic evaluation of an Nd:YAG laser-assisted new attachment procedure in humans. Int J Periodontics Restorative Dent. 2007;27(6):577–87.
20. Ozen T, Orhan K, Gorur I, et al. Efficacy of low-level laser therapy on neurosensory recovery after injury to the inferior alveolar nerve. Head Face Med. 2006;2:3. doi:10.1186/1746-160X-2-3
21. Ting CC, Fukuda M, Watanabe T, et al. Morphological alterations of periodontal pocket epithelium following Nd:YAG laser irradiation. Photomed Laser Surg. 2014;32(12):649–57. doi:10.1089/pho.2014.3793
22. Grimm WD, Dannan A, Becher S, et al. The ability of human periodontium-derived stem cells to regenerate periodontal tissues: a preliminary in vivo investigation. Int J Periodontics Restorative Dent. 2011;31(6):e94-e101. https://pubmed.ncbi.nlm.nih.gov/22140674/
23. Kim IS, Cho TH, Kim K, et al. High power-pulsed Nd:YAG laser as a new stimulus to induce BMP-2 expression in MC3T3-E1 osteoblasts. Lasers Surg Med. 2010;42(6):510–8. doi:10.1002/lsm.20870
24. Gkogkos AS, Karoussis IK, Prevezanos ID, et al. Effect of Nd:YAG Low Level Laser Therapy on Human Gingival Fibroblasts. Int J Dent. 2015;2015:258941. doi:10.1155/2015/258941
25. Crea A, Deli G, Littarru C, et al. Intrabony defects, open-flap debridement, and decortication: a randomized clinical trial. J Periodontol. 2014;85(1):34-42. doi:10.1902/jop.2013.120753
26. Verdugo F, D’Addona A, Pontón J. Clinical, tomographic, and histological assessment of periosteal guided bone regeneration with cortical perforations in advanced human critical size defects. Clin Implant Dent Relat Res. 2012;14(1):112–20. doi:10.1111/j.1708-8208.2009.00235.x
27. Reddy TS, Shah NR, Roca AL, et al. Space maintenance using tenting screws in atrophic extraction sockets. J Oral Implantol. 2016;42(4):353–7. doi:10.1563/aaid-joi-D-15-00048
28. Aoki A, Mizutani K, Schwarz F, et al. Periodontal and peri-implant wound healing following laser therapy. Periodontol 2000. 2015;68(1):217–69. doi:10.1111/prd.12080
ABOUT THE AUTHORS
Dr. Lehrman is a periodontist with practices both in New York City and Southampton, NY. Upon completion of his postgraduate training and his exposure to laser-assisted regenerative surgery in 2006, Dr. Lehrman has dedicated his clinical practice to the treatment of periodontal, peri-implant, and cosmetic periodontic cases with a laser-assisted approach. He is engaged in both clinical and laboratory research with an eye toward creating “wound healing” with the advent of the Nd:YAG wavelength. He can be reached at nl@nldds.com.
Dr. Drew is a professor, a director of implantology, and the vice chairman in the Department of Periodontics at the Rutgers School of Dental Medicine (RSDM). He received his doctorate and degree in periodontics from RSDM. He has been awarded the RSDM Excellence in Teaching Award, the Stuart D. Cook Master Educators Guild Award, and the prestigious AAP Educator Award. Dr. Drew was inducted into the American College of Dentists, and he was awarded the RSDM Alumni Association Decade (1980s) Award. He has written more than 25 articles for publications and has lectured throughout the country. He was in full-time clinical practice for more than 25 years. He can be reached at drhjdrew@aol.com.
Dr. Strauss is a board-certified periodontist with a private practice in Englewood, NJ, limited to periodontics and implantology. He received his doctorate in dental medicine from the University of Pennsylvania where he graduated with clinical honors and completed his postgraduate training in periodontics at Rutgers University, where he received multiple honors and awards, including the Balbo clinical poster award. He has presented and published articles on surgical canine exposures, periosteal pouch bone grafting, implant osteotomy preparation, and the treatment of peri-implantitis. At the 2018 Greater New York Dental Meeting, Dr. Strauss received the prestigious Carl Misch award for his excellence in presenting on the topic of alternative bone grafting techniques. He can be reached at gabriel.m.strauss@gmail.com.
Dr. Rynar is a graduate of Brown University and received his DMD degree and Certificate of Advanced Graduate Study in Periodontology at Boston University. He is a clinical professor of periodontics at RSDM. He is a certified Institute for Advanced Laser Dentistry Instructor. He has lectured throughout North America and Europe on periodontic and implantology topics, including immediate loading, implant concerns in soft bone, and periodontal/implant aesthetics. He has been published in various periodontal journals. Dr. Rynar practices in Florham Park, NJ. He can be reached at jamesrynar@gmail.com.
Dr. Kashikar is a board-certified oral pathologist with a doctorate in dentistry from the New York University College of Dentistry. She completed her residency at the NewYork-Presbyterian Queens Hospital in Queens, NY. Dr. Kashikar completed a one-year fellowship in dental oncology at Memorial Sloan Kettering Cancer Center in New York City. She has taught as a professor at RSDM for the last 3 years and was interim director of the biopsy service at the school. She is currently pursuing other interests and maintains her board status in oral pathology. She can be reached at skashikar86@gmail.com.
Disclosures: Dr. Lehrman has served as an instructor at the Institute for Advanced Laser Dentistry (IALD), but currently does not receive compensation from Millennium Dental Technologies, Inc. Drs. Lehrman and Strauss have received honoraria for lectures for the IALD. All other authors report no disclosures.

