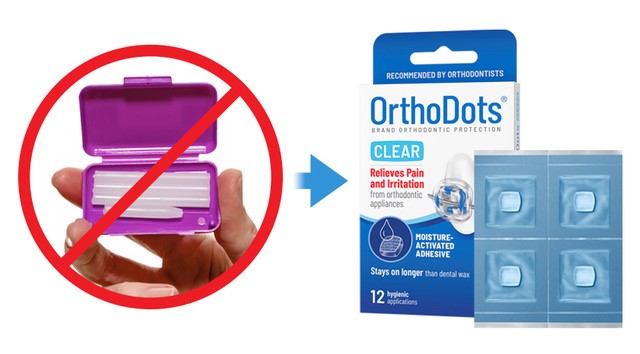
Dubbed “Ax the Wax,” the campaign follows research by Michigan State University advertising classes highlighting the importance of educating parents on the deficiencies of dental wax and a Coalition Letter to the FDA requesting action against the companies that continue to sell misbranded dental wax to orthodontic practices.
Click to view Ax the Wax video
According to OrVance chief executive officer Ron Schutt, “Although this ad campaign is directed to orthodontic patients and their parents, it is also intended to call on the identified companies that continue to sell this product to do the right thing. In a Coalition Letter to the FDA back in May, we have identified 25 companies that continue to sell misbranded dental wax. This letter has been uncontested and not one of these companies has stepped forward to defend why the dental wax they sell should be exempt from current product safety and regulatory standards. We need more accountability against the identified importers and distributors that continue to push this substandard product throughout our industry.”
Dr. Eric Hannapel, orthodontist and OrVance co-founder, added, “Dental wax is still the most commonly dispensed product in our profession where most of our patients are children, so it’s unfortunate that we need a consumer advertising campaign to bring this issue to light. We believe it’s time these companies either address their noncompliance or explain to practices and the public why they believe this product doesn’t need to meet such basic product safety standards such as hygienic packaging, tamper evidence, disclosure of ingredients, product traceability and FDA required labeling. On a positive note, I want to applaud industry leaders such as GC Orthodonticsthat are partnering with us to fund the replacement of noncompliant dental wax for the health and safety of our patients.”
As part of the campaign, OrVance is encouraging patients and parents to ask their orthodontist about OrthoDots® CLEAR so they can experience the many performance and quality benefits the product offers compared to traditional dental wax. Orthodontic practices can order free samples of OrthoDots® CLEAR at orvance.com.
Contact:
Craig Clark,
(616) 550-2736,
craig@clarkcommunication.com
About OrVance
OrVance LLC is a developer of proprietary oral health products and is based in Grand Rapids, Michigan. It is over 90% owned by orthodontists, dentists and its managing partners. Learn more at orvance.com.