Chemicals
Solvents were of LC-MS grade (Honeywell Riedel-de Haën, Seelze, Deutschland). Water was prepared by a Milli-Q apparatus (Millipore, Schwalbach, Germany). Chemicals were purchased from Sigma-Aldrich (Steinheim, Germany) and Merck (Darmstadt, Germany). Stable isotope-labelled compounds were purchased from Cambridge Isotope Laboratories (Radeberg, Germany) and Sigma-Aldrich. Artificial saliva for method development and validation was prepared according to the literature29. In addition, artificial saliva was prepared without α-amylase for method development of carbohydrates, because α-amylase gave a blank value for glucose and maltose of substantial peak area.
Subjects
A total of 57 children (33 female and 24 male volunteers ages 4–6 years) participated in the present study. Saliva for the analysis of amino acids, carbohydrates, and organic acids was collected from all subjects, in which 18 were caries free with no decayed, missing, or filled teeth (dmf/t = 0), 18 had dental restorations (dmf/t ≥ 2; no active carious lesions), and 21 were caries active (dmf/t ≥ 2; at least two carious lesions). Pellicle samples were collected from 40 children out of the panel, in which 17 were caries free (dmf/t = 0), 12 had dental restorations (dmf/t ≥ 2), and 11 were caries active (dmf/t ≥ 2). The analysis of long-chain fatty acids was performed in saliva and pellicle samples of 40 children, in which 16 were caries free (dmf/t = 0), 13 had dental restorations (dmf/t ≥ 2), and 11 were caries active (dmf/t ≥ 2). Oral examination was conducted by a dentist experienced in paediatric dentistry. The following indices were determined: dmf/t, dmf/s, QHI and GI.
See Table 1 for details on the study groups. The study design was approved by the Ethics Committee of the Technische Universität Dresden (EK #79032012, Ethics Committee of the Medical Faculty, TU Dresden) before recruitment and study initiation. The study was performed according to the guidelines of the Declaration of Helsinki. The clinical part and the acquisition procedures of saliva and pellicle samples, respectively, were checked and approved by the local ethics committee. Informed written consent was obtained from all participants and their legal guardians.
Saliva and pellicle collection
The parents were asked to clean their children’s’ teeth in the morning and 30 min before pellicle collection by tooth brushing without toothpaste, and application of dental floss. Around 10:00 am unstimulated saliva was collected under medical supervision by spitting in a sterile tube. The samples were immediately frozen −20 °C and then stored at −80 °C until further sample preparation.
For pellicle collection, ceramic specimens were fixed to “lollipops” made of two-component silicone (nine specimens per lollipop). The lollipops were placed in the buccal cavity in the lower jaw, in which the specimens were faced towards the tongue. In this way, three lollipops were incubated in the oral cavity one after the other for 10 min each for pellicle formation. The acquired pellicle was desorbed by working at a clean bench with sterile materials using an in-house-developed protocol. Triton-X buffer consisting of 10% 10 × Tris-HCl-NaCl buffer (0.2 M Tris, 1.5 M NaCl, pH 7.5), 20% protease inhibitor (cOmplete, EDTA free; Roche, Mannheim, Germany), and 1% Triton-X in bidistilled H2O and RIPA buffer consisting of 10% 10 × RIPA buffer (Cell Signaling Technologies, Frankfurt, Germany) and 20% protease inhibitor (cOmplete, EDTA free, Roche, Mannheim, Germany) in bidistilled H2O were prepared for desorption. The specimens were rinsed with ultrapure water (20 mL) to remove loosely attached salivary components. Pellicle was subsequently desorbed by rinsing with Triton-X buffer (700 µL) and RIPA buffer (700 µL). Both solutions were pooled and stored at −80 °C until further sample preparation. Pellicle collection for the analysis of long-chain fatty acids was done according to the literature with a pellicle formation time of 30 min followed by desorption with 0.4% EDTA solution30,46.
The collection of pellicle and saliva samples was done during a single session. During the session, participants refrained from eating and drinking.
Quantitative analysis
Calibration standards for amino acids, organic acids, and carbohydrates were prepared individually per compound class from diluted mixtures of stock solutions of the unlabelled substances and mixed with a fixed amount of labelled compound as the IS according to the calibration ranges and protocols detailed below. Calibration standards were injected as triplicates. The area ratios of the analyte/IS were plotted against the concentration ratios followed by linear regression to create the calibration curves. For in-house validation, artificial saliva was spiked with a mix of the analytes in final concentrations of 1, 10, and 100 nmol/mL. The samples were processed in triplicate according to the respective quantitation protocol (see below). Each sample was analysed in replicates (n = 3), and to evaluate the technical precision, one sample was injected 10 times. The ratio of calculated to nominal concentration × 100% represents accuracy, and precision was expressed as relative SD (cf. Supporting Table 4).
The analysis of fatty acids in saliva and pellicle desorbates was performed as described in detail previously30,46.
Amino acids
Stock solutions of analytes and IS and calibration
Unlabelled amino acids were individually dissolved in 30% aqueous acetonitrile. Appropriate aliquots were combined to obtain a mixed analyte solution (1000 nmol/mL). Labelled amino acids were combined in a mixed IS solution with concentrations between 50 and 250 nmol/mL (see details in Supporting Table 8). Dilutions of the analyte solution with 90% acetonitrile were spiked with the IS solution (100 µL) to obtain final analyte concentrations of 100, 50, 25, 10, 5, 2.5, 1, 0.5, 0.25, 0.1, 0.05, 0.025, 0.01, 0.005, 0.0025, and 0.001 nmol/mL of each analyte with a fixed amount of IS.
Sample preparation for quantitation
Saliva samples and pellicle desorbates were centrifuged (10 min, 4 °C, 12,000 rpm) to separate mucins. The supernatant (100 µL) was mixed thoroughly with the IS (5–25 nmol/mL, 10 µL) in an Eppendorf cap (2 mL). Ice-cooled acetonitrile (390 µL) was added and the solution was mixed again. The precipitate was separated by centrifugation (10 min, 4 °C, 13,200 rpm), and the supernatant was transferred into another Eppendorf cap and evaporated to dryness (37 °C, 90 min, 35 kPa). The residue was solved in 50% aqueous acetonitrile (50 µL) and stored at −20 °C until measurement.
Instrumental analysis with UPLC-MS/MS
The amino acids were separated on an Acquity BEH amide column (1.7 µm, 2.1 × 100 mm, Waters, Eschborn, Germany) at a flow rate of 0.4 mL/min. Water containing ammonium acetate (5 mM) and formic acid (0.1%) was solvent A, and 95% acetonitrile with ammonium acetate (5 mM) and formic acid (0.1%) was solvent B. The gradient elution started with 90% solvent B for 0.02 min. Solvent B was decreased to 85% within 4.98 min and 70% within 3 min. Solvent A was increased to 100% within 1 min followed by isocratic elution (2 min). The re-establishment of the starting conditions was done within 1 min followed by re-equilibration time for 2 min. The UPLC System (Shimadzu Nexera X2; Shimadzu, Duisburg, Germany) was coupled to a 6500 Q-Trap MS (Sciex, Darmstadt, Germany) with Analyst 1.6.2. Ionisation was done in positive electrospray mode. The configuration was 35 psi for curtain gas, 5500 V for ion spray voltage, 55 psi for heater gas, and 65 psi for turbo gas. The source temperature was set to 400 °C. MS analysis was done by means of a scheduled MRM method. See Supporting Table 1 for MS/MS parameters.
Organic acids
Reagents, stock solutions of analytes and IS, and calibration curves
Stable isotope-labelled organic acids were mixed in 50% acetonitrile at a final concentration of 2000 nmol/mL. A mixture of unlabelled standards was prepared in 50% acetonitrile at a concentration of 2000 nmol/mL. The standard mixture of organic acids with a concentration of each analyte of 2000 nmol/mL was serially diluted 1:1 with water leading to solution from 1000 to 0.25 nmol/mL per analyte. Aliquots (50 µL) of each standard solution were mixed with the IS working solution (200 nmol/mL, 50 µL). This resulted in 14 standards with a fixed amount of each stable isotope-labelled standard and 1000, 500, 250, 125, 62.5, 31.25, 15.6, 7.8, 3.9, 1.96, 0.98, 0.49, 0.25, and 0.125 nmol/mL of each analyte. Solutions of 3-nitrophenylhydrazine (3-NPH; 200 mM in ACN/H2O) and N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide (EDC; 120 mM in ACN/H2O containing 6% pyridine) were prepared for derivatisation following the literature protocol25,26. Prepared calibration standards were treated similar to authentic saliva samples as described in detail below.
Sample preparation for quantitation
Saliva samples and pellicle desorbates were centrifuged (10 min, 4 °C, 12,000 rpm) and the supernatant (100 µL) was mixed with the IS solution (200 nmol/mL, 50 µL). Ice-cooled acetonitrile (350 µL) was added and precipitated proteins were removed by centrifugation (10 min, 4 °C, 13,200 rpm). Solutions of 3-NPH (20 µL) and EDC (20 µL) were added to the supernatant and incubated (30 min, 40 °C) after mixing. Water (460 µL) was added and the solution was centrifuged (3 min, 10 °C, 12,000 rpm). Note: The samples should be measured immediately or stored at −20 °C. Precipitates can occur upon storage, which need to be removed (e.g. centrifugation) before instrumental analysis.
Instrumental analysis with UPLC-MS/MS
For the chromatographic separation of organic acids, an Acquity BEH C18 column was used (1.7 µm, 2.1 × 100 mm; Waters, Eschborn, Germany). The flow rate was set to 0.4 mL/min, and 0.1% formic acid in water (solvent A) and acetonitrile (solvent B) served as solvents. The gradient started with an isocratic step at 7% solvent B (2 min) and increased to 45% (7.5 min) and then to 100% solvent B (0.5 min). After isocratic elution (1 min), the starting conditions we re-established (0.1 min) followed by the equilibration of the system at 7% solvent B (2.9 min). The UPLC System (Shimadzu Nexera X2, Shimadzu, Duisburg, Germany) was coupled to a 5500 Q-Trap MS (Sciex, Darmstadt, Germany) with Analyst 1.6.2. Ionisation was performed in negative electrospray mode. The setting was 35 psi for curtain gas, −4500 V for ion spray voltage, 55 psi for heater gas, and 65 psi for turbo gas. The source temperature was set to 500 °C. MS/MS analysis was done in MRM mode, in which each transition was recorded with a dwell time of 5 ms. See Supporting Table 2 for MS/MS parameters.
Carbohydrates
Reagents, stock solutions of analytes and IS, and calibration curves
The IS 13C6-glucose and 13C5-ribose were mixed in 50% aqueous acetonitrile to a final concentration of 50 nmol/mL. The unlabelled compounds were mixed in 50% aqueous acetonitrile at a final concentration of 2000 nmol/mL. The analyte solution was appropriately diluted with 10% acetonitrile to 500, 100, 50, 10, 5, 1, 0.5, 0.1, 0.05, 0.01, 0.005, and 0.001 nmol/mL of each analyte. Aliquots (100 µL) were mixed with the IS solution (5 µL). This resulted in 12 standards with a fixed amount of each stable isotope-labelled standard. Each standard solution was derivatised. 2-Anthranilic acid (2-AA; 0.35 M in DMSO containing 15% glacial acetic acid) and 2-picoline borane complex (2-PB; 1 M in DMSO) were prepared for derivatisation28.
Sample preparation for quantitation
Saliva samples were centrifuged (10 min, 4 °C, 12,000 rpm) and a mixture of stable isotope-labelled standards (50 nmol/mL, 5 µL) was added to the supernatant (100 µL). By adding ice-cooled acetonitrile (395 µL) and centrifugation (10 min, 4 °C, 13,200 rpm), proteins were precipitated and separated. The supernatant was transferred into another Eppendorf cap and evaporated to dryness (37 °C, 70 min, 35 kPa). The residue was solved in 10% aqueous acetonitrile (100 µL) and mixed with solutions of 2-AA (5 µL) and 2-PB (5 µL) for derivatisation. After the reaction (2 h, 65 °C), the mixture was centrifuged (10 min, 10 °C, 13,200 rpm), and the supernatant was transferred into a vial and stored at −20 °C until measurement.
Centrifuged pellicle desorbate (100 µL) was spiked with the IS solution (50 nmol/mL, 5 µL) and mixed with ice-cooled acetonitrile (395 µL). After centrifugation (10 min, 4 °C, 13,200 rpm), the supernatant was evaporated, and the residue was taken up in 10% aqueous acetonitrile (200 µL) and derivatised. After the reaction (2 h, 65 °C), the turbid solution was transferred into centrifugal filter units (Amicon Ultra, 0.5 mL Centrifugal Filters, Ultracel 3 K, Regenerated Cellulose; Merck) and centrifuged (10 min, room temperature, 12,000 rpm). The clarified supernatant was transferred into a vial and stored at −20 °C.
Instrumental analysis with UPLC-MS/MS
Separation was achieved with a Kinetex F5 column (1.7 µm, 2.1 × 100 mm; Phenomenex, Aschaffenburg, Germany). The flow rate was set to 0.4 mL/min, and 0.1% formic acid in water (solvent A) and acetonitrile (solvent B) were used as solvents. The gradient started at 5% solvent B and increased to 6% (6 min) and then to 100% solvent B (1 min). After isocratic elution (2 min), the re-establishment of the starting conditions was done (0.5 min) followed by an equilibration time of 3.5 min at 5% solvent B. The UPLC System (Shimadzu Nexera X2, Shimadzu, Duisburg, Germany) was coupled to a 5500 Q-Trap MS (Sciex, Darmstadt, Germany) with Analyst 1.6.2. Ionisation was performed in negative electrospray mode. The setting was 35 psi for curtain gas, −4500 V for ion spray voltage, 55 psi for heater gas, and 65 psi for turbo gas. The source temperature was set to 500 °C. For MS/MS analysis, the MRM mode was used, in which each transition was recorded with a dwell time of 20 ms. See Supporting Table 3 for MS/MS parameters.
Statistics
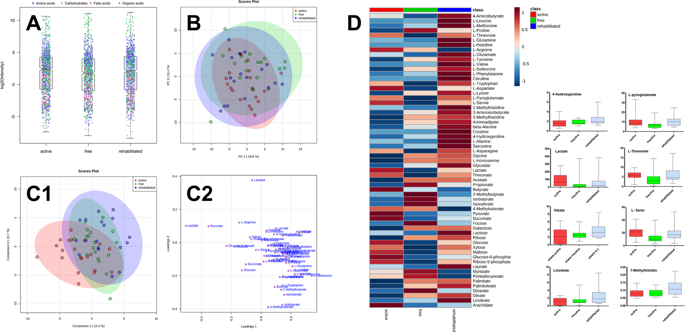
Data handling was done using Microsoft Excel 2016, GraphPad 5.0 for Windows (GraphPad Software, San Diego, CA, USA), and MetaboAnalyst 4.0 (https://www.metaboanalyst.ca/)48,49,50. For statistical evaluation, analytes that could be quantified (S/N > 10) in more than 80% of the samples per group were used. The data were log transformed. To evaluate the significant differences among the caries-free, caries-rehabilitated, and caries-active groups, univariate analysis was performed by one-way ANOVA. The FDR was set to default (0.05). Further information is found in the figure descriptions.