Study design and ethical considerations
This was a single blind-randomized control trial with a two-arm parallel superiority design with a 1:1 allocation which was conducted during the period between October 2021 to October 2023 in the Department of Pediatric Dentistry at the Faculty of Dentistry, Damascus University, Syria.
All procedures in this study were in accordance with the declaration of Helsinki guidelines and its Consort recommendations and received ethical approval from the Local Research Ethics Committee of the Faculty of Dentistry [Approval No. UDDS-502-220420121/SRC-3183]. The research was registered on clinicaltrials.gov [NCT05759286] and the informed consent was taken from the Participant’s parents or legal guardians before the procedures were carried out and after an adequate explanation was provided about this study and the procedures that will be done.
Sample and sampling methods
The sample size was calculated using G* Power 3.1.9.4 [Heinrich-Heine-Universität, Düsseldorf, Germany], based on the effect size of the previous study with pulse rate’s Means ± SD in control and experimental groups were 10.8 ± 7.5, −8.3 ± 6.8, respectively [26]. The adequate total sample size for two groups at the effect size f = 0.34/α err type = 0.05/and study power [1−β] err prob = 85% was 56 children.
Recruitment and eligibility criteria
Inclusion criteria
- 1.
Healthy children with no systematic diseases.
- 2.
Children aged between 6 and 10 years old.
- 3.
Children without previous dental history.
- 4.
Children who recorded 2 grade [positive] on the Frankle behavior scale.
- 5.
Children who needed dental treatment in mandibular teeth requested anesthesia with alveolar nerve block injection.
- 6.
Children with sufficient cognitive skills to complete the self-report scale.
Exclusion criteria
- 1.
Children whose parents refused to participate in this study.
- 2.
Children with mental and physical disabilities.
- 3.
Children with colds, asthma, and any other respiratory diseases.
- 4.
Children with acoustic problems.
- 5.
Children who are allergic to any of the essential oils that have been used in this study.
- 6.
Children who took NSAIDs or analgesics drugs in the last 8 h before treatment
70 children were assessed for eligibility, 14 of them were excluded due to not meeting the inclusion criteria. Finally, fifty-six children were included in this study who were randomly divided into two groups at an allocation ratio of 1:1 with a simple randomization method using the www.random.org website (Fig. 1), which was accessed on 10 October 2021. Thus, children were assigned to two groups: Group 1: control group [n = 28], and Group 2: aromatherapy with music group [n = 28].
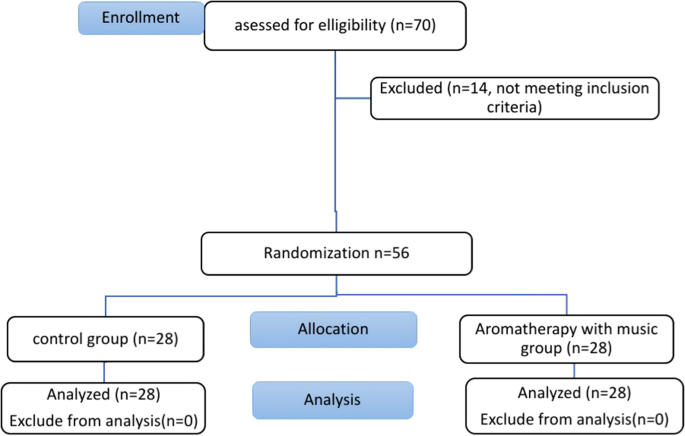
Modified Consort flowchart.
Intervention
After taking demographic information and informed consent from parents, the children were randomly divided into two groups.
Aromatherapy combined with a music group
The Lavender-Neroli oil blend was first prepared in Damascus University’s Faculty of Pharmacology Department of Pharmacognosy by blending 2.3 ml Lavender oil [100% L. angustifolia essential oil] with 0.9 ml Neroli oil [100% C. aurantium essential oil], and the final blend was diluted into 20 ml using grape seed oil as a carrier oil. The essential oils were obtained from Biocham natural extract.co, Damascus, Syria, and the main components were determined by Gas Chromatography [GC] which found to be 37% linalool, 11.6% camphor, 9.9% 1.8 cineole, 5.5% linalyl acetate in Lavender oil, and 23.4% linalool, 15.5% linalyl acetate,12.3% trans-nerolidol, 11.9% limonene, 7.7% β pinene in Neroli oil, these data were provided by the delivering company.
In the dental chair, the child was asked to inhale the aromatic blend and listen to self-choose music for 5 min before and during anesthesia. Aromatherapy was done by informing the child to wear a nitrous oxide nasal mask [Accturon, Hu-Friedy Mfg. Co., LLC] after modifying it by putting a 3D printed box which was perforated from its bottom and top on its circle hole, and three drops of the aromatic blend were poured on three cotton balls which were put inside the box’s cavities (Fig. 2A–D) Music in this study was done by using a wireless speaker that was connected to a mobile phone through Bluetooth and the child was asked to choose his/her favorite music from their cartoons and program on YouTube and then listen to it as a background in the treatment room (Fig. 2D).

Modified nitrous oxide nasal mask, (A) 3D printed box, (B) placement of the cotton balls inside box’s cavities, (C) the modified nasal mask after the 3D printed box was patterned on its circle hole, (D) A child inhale the aromatic oils from the modified nasal mask and listening to music from a speaker.
Control group
Children in this study were informed to wear another modified nitrous oxide nasal mask with an empty 3D printed box was put on its circle hole as a placebo during IANB injection.
Children in both groups received IANB injections by the same research using lidocaine 2% with 1:80,000 epinephrine [Huons Lidocaine HCL, Seoul, Korea] and a 27-gauge needle [Kohope, Shanghai, China], which was used at a depth of 15 ml where 1 ml of the anesthetic’s solution was injected for 1 min [27].
Outcomes and measurements
Dental anxiety and pain were defined as the first outcome measures and changes in physiological parameters, including heart rates, SPO2 saturation, diastolic and systolic blood pressure were considered the second.
Assessment of dental anxiety was conducted using FIS, it consisted of five faces ranging from very happy to very sad, and the child was asked to point at the face that mostly reflected his/her feelings. The score of this scale ranges from being 1 to the most positive face to being 5 to the most negative face [28, 29].
Pain assessment during anesthesia injection was done using the Arabian version of the Face-Legs -Activity-Cry-Consolability [FLACC] objective pain scale, it is considered a reliable and valid tool to assess pain in children aged 6–14 years old [30]. It includes five categories of pain behavior and each category has a score between 0 and 2, which makes the whole scale’s scores range between 0 and 10 [31].
FLACC assessment was done by using a video filmed of the child’s behavior during anesthesia and then all videos were evaluated by three trained residents in the Pediatric Dentistry Department at Damascus University who had dealt previously with the FLACC scale in their research and they were blind to the procedures in each group as the children in both groups were wearing the nasal mask with the box on it and the music sound was pulled from the video during evaluation.
physiological parameters including diastolic and systolic blood pressure, heart rate, and SPO2 saturation were assessed before and after 1 min from anesthesia injection, Heart rate and SPO2 saturation were recorded using a pulse oximeter [Meditech Equipment, Shandong, China], and diastolic and systolic blood pressure were recorded through digital blood pressure monitor with an elbow cuff [O2 medical systems, Hyderabad, India].
Statical analysis
The data of the present study were analyzed using the SPSS 21.0 software (IBM, Armonk, NY, USA). Statistical analysis was performed using the SPSS 21.0 software (IBM, Armonk, NY, USA). The data were analyzed with Mann Whitney U, Wilcoxon signed-rank test, paired t-test, and Independent-T test. The testing was performed at α = 0.05.

