This is a double-blind, randomized, crossover, single center, controlled in situ study to establish the equivalence of two children toothpaste formulations, containing either 10% HAP microclusters (crystallite size: length ≈ 80 nm (median) × width ≈ 30 nm (median)) or 500 ppm fluoride provided as amine fluoride (AMF), in inducing the remineralization and inhibiting the development of initial caries lesions. The primary outcomes to be examined were (1) the percentage remineralization and lesion depth reduction measured relative to the baseline mineral loss and lesion depth for initial caries, and (2) the amount of mineral loss and lesion depth for the sound enamel. The study was conducted at the clinical research facility of the University of Texas Health San Antonio (UTHSA) School of Dentistry. The UTHSA Institutional Review Board (IRB) approved the study (protocol #: HSC20180416H), and the study was registered with ClinicalTrials.gov (NCT03681340). The study was conducted in accordance with the ethical standards outlined in the 1964 Declaration of Helsinki and its later amendments, and in compliance with the International Conference on Harmonization (ICH) Good Clinical Practice Guidelines. The participants were recruited from among different ethnic origins and varied socio-economic status in the local San Antonio area, written informed consent was obtained from all participants prior to their participation in the study.
Participant recruitment
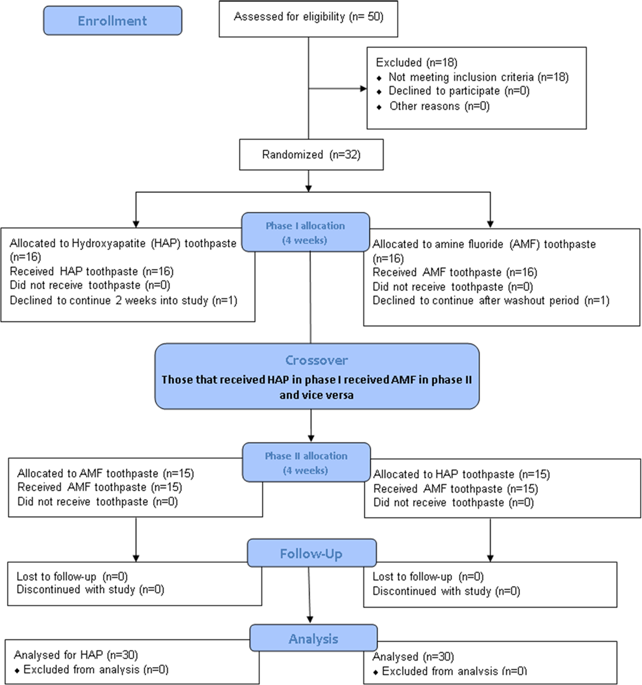
Fifty subjects aged from 18 to 60 years were given screening examination that included sialometry, medical/dental history, and oral examination (Fig. 1). Thirty two subjects qualified and were enrolled in the study. Inclusion criteria were age of 18 through 60 years; normal salivary function with unstimulated and stimulated salivary flow rates ≥0.2 ml/min and ≥0.7 ml/min, respectively, measured according to the Sreebny and Valdini33 procedure. Other inclusion criteria were not taking any antibiotics or medications which could affect saliva flow rate; the presence of at least 20 natural uncrowned teeth (excluding third molars); a past history of dental caries but no clinically active caries; willing to wear the in situ appliance and use only assigned products for oral hygiene throughout the duration of the study; the ability to read and understand English; ability to provide informed consent; and no self-reported history of allergy to personal care/consumer products or any ingredient in the test products. Exclusion criteria were the presence of advanced periodontal disease or other oral pathology; medical condition that requires premedication prior to dental procedures; use of antibiotics one month prior to or during this study; self-reported pregnancy or breastfeeding; and use of tobacco products.
Flow Diagram detailing the stepwise methodology. This is a crossover study so the 30 completers received the two intervention in a crossover design as phase I and II.
Creation of artificial initial caries and fabrication of the in situ appliance
Following consent from the donors, freshly extracted primary teeth were collected from the pediatric clinics of the UTHSA School of dentistry and stored in 0.1% thymol solution at 4 °C prior to use. The teeth were examined with a transilluminator, and thirty two teeth without caries, cracks, or enamel malformations were selected and cleaned with pumice using electronic toothbrush. Using a water-cooled diamond wire saw, 4 tooth blocks were produced from buccal and lingual surfaces of each of the selected teeth, with each block measuring ~2 mm length × 2 mm width × 1.5 mm thickness. Two of the 4 blocks were retained as sound enamel blocks for demineralization inhibition assessment while the other two blocks targeted for remineralization assessment had artificial initial caries produced in them as follows. All surfaces of each block were painted with two coats of acid resistant nail varnish except buccal or lingual on which an initial caries lesion was created by subjecting this exposed surface to 7 days demineralization in an acidified gel system (0.10 M lactic acid, 0.10 M sodium hydroxide, 6% w/v hydroxyethyl cellulose, pH 4.5). Following lesion formation, the nail varnish was carefully removed with acetone. A tooth section (~150 µm thick) was cut from each tooth block for the measurement of the baseline mineral loss (∆z1) and lesion depth (LD1) of each produced initial caries lesion, and for selection of the suitable lesions for the remineralization assessment. The sections were processed for transverse microradiography (TMR) as follows. Both sides of the sections were polished using adhesive back lapping film in a MultiPrep™ Precision Polishing machine (Allied High Tech, USA) to achieve planoparallel surfaces, as well as reduce the thickness of the slice to 100 µm (the appropriate thickness for TMR). Following this, the sections were microradiographed on a type lA high resolution glass X-ray plate (Microchrome Technology, CA, USA) using a Phillips X-ray generator system set up for this purpose. The plates were exposed for 10 min at an anode voltage of 20 kV and a tube current of 10 mA, and then processed. Processing consisted of a 5 min development in Kodak HR developer and 15 min fixation in Kodak Rapid-fixer before a final 30 min wash period. After drying, the microradiographs were examined under a Leica DMR optical microscope linked via a Sony model XC-75CE CCTV camera to a personal computer. Using TMR2006 version 3.0.0.11 image analysis software (Inspektor Research Systems, Amsterdam, Netherlands), the enhanced image of the microradiographs were analyzed under standard conditions of light intensity and magnification along with the image of a step wedge as described by de Josselin de Jong et al.34 At this point, the images were used only for selection of the suitable lesions for the study. Only the controls that showed caries-like lesions with subsurface lesions, which display a fairly uniform width throughout their length, were selected for the remineralization process, and their test blocks were used for construction of the in situ appliance. It is pertinent to mention that the baseline measurements were not conducted for the sound tooth blocks to be used for demineralization-prevention study because the TMR does not measure the mineral density of sound tooth tissue rather the software uses the known mineral density of sound enamel or dentin to determine the amount of mineral loss in a demineralized tissue.
As stated above, the four tooth blocks from each tooth were distributed as follows: two lesion-bearing blocks for remineralization assessment and two sound blocks for demineralization inhibition assessment. These four blocks were used to fabricate the in situ appliances as follows. Each block was covered with polyester gauze (Bard Peripheral Vascular, Inc., Tempe, AZ, USA) and mounted within an in situ appliance, a customized orthodontic bracket.27 The polyester gauze facilitated plaque retention on the surface of the tooth blocks on intra-oral exposure. The appliance consists of an orthodontic molar pad with retentive mesh backing (American Orthodontics Corp., Sheboygan, US), which has a ring of 0.7 mm orthodontic wire welded to it so that the ring closely encircles each test-block. The block was retained within the bracket with fluoride-free intermediate restorative material (IRM). All appliances were sterilized with gamma irradiation prior to delivery to the subject.
Study treatment
The study was performed in two distinct treatment phases during which subjects were exposed to one of the following two treatments in a randomized crossover design; (A) Karex Kid’s toothpaste containing 10% HAP microclusters (Kinder Karex, Dr. Kurt Wolff GmbH and Co. KG, Bielefeld, Germany), and (B) Elmex Kid’s toothpaste containing 500 ppm fluoride as AMF (Elmex Kinder zahnpasta, GABA GmbH, Hamburg, Germany). Each phase started with one week of washout period and then 4 weeks of treatment, consisting of two 2-week periods during which each subject used his/her assigned treatment under the following conditions. First 2-week period, with the subject wearing an in situ appliance with sound enamel block, and the second 2-week period, with the subject wearing an in situ appliance with lesion-bearing tooth block.
Subjects who satisfied enrollment criteria were given a specially manufactured washout toothpaste with neither fluoride nor HAP (Dr. Kurt Wolff GmbH and Co. KG, Bielefeld, Germany) and an adult soft-bristled toothbrush to use for a washout period of one week. The washout period allows for attenuation of any residual effect of the subject’s previously used toothpaste. There was no washout period between individual 2-week treatment periods within each phase since the subjects used the same product for the 4 weeks. During the washout period, subjects were instructed to use the toothpaste and toothbrush for 2 min twice a day (morning after breakfast and night last thing before bed) in place of their normally used toothpaste and toothbrush, and as their only oral hygiene product. Subjects were given no restriction on dietary habit.
At the end of the washout period, patients returned to the clinic, and were assigned to a group to use either HAP or AMF by the Study Coordinator using randomization numbers generated by a computer program designed and operated by our biostatistics team. However, to ensure that both the operators and the subjects were blinded as to product assignment, all toothpaste tubes were packaged identically and coded (A, B, or washout) by the manufacturing/packaging company, who retained the code until the completion of the study and data interpretation. Following randomization, the 4 block-bearing in situ appliances originating from one tooth were assigned to one subject. Then the first of the four assigned appliances was bonded, in accordance with current principles of orthodontic practice, on the buccal surface of the chosen lower molar tooth. The appliance was bonded by a qualified dentist licensed in the state of Texas, who was different from the laboratory technician that process and analyze the samples to produce the final data. To bond the appliance, the buccal surface of the tooth chosen was carefully etched for 30 s, washed with water spray and dried for a further 30 s, and isolated using cotton rolls. The bottom of the appliance was loaded with Transbond™ XT light-cure adhesive paste (3M Unitek, Monrovia, CA, USA), and carefully positioned to avoid causing occlusal interference and to avoid soft tissue irritation. The excess composite material that spilled out from the sides of the appliance was used to cover the sides, beveling it to present a comfortable streamlined (non-catching) surface when the slab comes in contact with a soft tissue surface (e.g., tongue). The adhesive paste was cured using an Ortholux XT visible Light Curing Unit (3M Unitek, Monrovia, Ca, USA) applied for 20 s.
Following bonding of the appliance, each subject was given his/her respective test toothpaste and a soft-bristled toothbrush designed for use with orthodontic brackets with shorter bristles at the center to accommodate the bracket. Subjects were instructed to continue with the routine of brushing two times daily, morning after breakfast and last thing before bed, for 2 min before rinsing with only 10 ml of water. Subjects were also given special instruction on dispensing of the toothpaste, and were advised not to brush directly on the appliance but rather to brush around it to prevent disruption of the dental plaque on the surface of the tooth block. Subjects were restricted from eating nor drinking for at least 30 min after brushing. A timer and measuring cup were provided to each subject. As a method of monitoring compliance, a diary was provided to each subject for recording the time of each brushing episode, and in addition, toothpaste tubes were weighed at the time of randomization and at each study visit. Subjects were instructed to maintain their normal dietary habits and were prohibited from using any other oral hygiene product (e.g., mouthwash, chewing gum) or tooth-whitening product for the duration of the study. Immediately after bonding of the first appliance, each subject used the test product under the supervision of the Study Coordinator, and for the remainder of each treatment phase, subjects completed the procedure at home and as instructed by the Study Coordinator
Subjects returned to the clinical research facility after 2 weeks without using the product that morning, and the first appliance was detached and sent to the laboratory for analysis. The appliance for the second 2-week treatment period of the phase was bonded, the dairy checked, the toothpaste weighed, and safety evaluation performed. Upon completion of the second 2-week treatment period, the subject again returned to the clinic for detachment of the second appliance, and was given washout toothpaste and a soft-bristle toothbrush to undergo another 7-day washout period without an appliance in preparation for his/her phase 2 of the study. After completion of the washout period without an appliance, subjects return to the clinic, and the procedure of phase 1 was repeated until the second 2-week treatment period was completed, and each subject has gone through the two arms of the study.
Safety monitoring
At all visits, the dental examiner visually examines the oral cavity and peri-oral area, and this examination included an evaluation of the soft and hard palate, gingival mucosa, buccal mucosa, mucogingival fold areas, tongue, sublingual and submandibular areas, salivary glands, and the tonsillar and pharyngeal areas. In addition to oral examination, subjects were screened for adverse events using a questionnaire.
Post-treatment processing and study outcomes
Following intra-oral exposure, a tooth section (~150 µm thick) was cut from each tooth block, both sound and lesion-bearing blocks. The sections were processed for microradiography as described above for the control sections used for baseline data. Although the lesion-bearing control sections have been microradiographed and analyzed for selection of the appropriate lesions, they were microradiographed again together with the post-test sections and both analyzed together for quantification of the ∆z and LD of the lesions as described for baseline sections. This step enabled both control and test sections from same block to be microradiographed and analyzed under the same conditions. For the lesion-bearing sections, this process yielded the pre-test mineral loss (∆z1) and lesion depth (LD1), the post-test mineral loss (∆z2), and lesion depth (LD2), and the pre-test and post-test microradiograms of the lesions. For the sections from sound tooth blocks, the process yielded the post-test mineral loss (∆z) and lesion depth (LD) if any lesion developed, and the microradiograms. Using the microradiograms, the pattern and the extent of remineralization produced within each lesion by each treatment arm was examined by comparing the pre-test and post-test images side-by-side. For each participant the post-treatment mineral loss was subtracted from the pre-treatment mineral loss, and then standardized across participants by dividing that difference by the pre-treatment mineral loss to obtain the % remineralization. The lesion depth pre-treatment and post-treatment was handled the same way to obtain the % lesion depth reduction. The two products were compared using the % remineralization and the % lesion depth reduction.
Power analysis and sample size calculation
The sample size calculations, which were based on a power analysis, were performed using nQuery Advisor software (Statistical Solutions, Cork, Ireland). Based on previous studies in which the mean % remineralization was equal to 30.3 with a standard deviation equal to 16.3,27,35,36,37 and for the hypothesis that each of the two toothpaste formulations promotes remineralization and lesion depth reduction that is significantly greater than zero, an effective sample size of 30 subjects will have power greater than 0.95 with a 0.05 one-sided significance level to detect a difference between a hypothesis mean of zero and a sample mean % remineralization equal to or greater than 10% using a two-sided t-test of two independent means. However, 32 subjects were enrolled to make provision for 5% dropout.
Statistical analysis
For measurements by both mineral loss and lesion depth, three endpoints were analyzed. (1) The mean amount of remineralization and mean amount of lesion depth reduction were determined for Karex toothpaste as a percentage of pre-treatment mineral loss and pre-treatment lesion depth respectively, and these percentages were compared to a value of 0%, which is what would be expected if the toothpaste had no effect. The statistical test used was a one-sided t-test of one group mean. (2) In the same way, the mean amount of remineralization and mean amount of lesion depth reduction was determined for Elmex toothpaste, and also compared to 0%. (3) Finally, the primary endpoint was to compare the means of Karex toothpaste to Elmex toothpaste to check for non-inferiority/equivalence of the HAP toothpaste to the fluoride toothpaste, using the two-sided t-test of two independent means. Non-inferiority/equivalence was established if the difference between the two toothpaste formulations for any one measurement method was not regarded to be clinically relevant and was set to Δ ≤ 20%. The statistical package R, version 3.5.0, was used for analysis.