Sample preparation
Human third molars from 18–35 years-old healthy patients were extracted (n = 10). This procedure was approved by the Ethics Committee of our Institution (University of Valencia, registration number: H1443515306255). Additionally, all methodology used was carried out in agreement with the national clinical guidelines.
Teeth were washed to remove some organic remains, immersed in a 0.1% thymol solution at 4 °C for 48 h and finally placed in sterile distilled water at r/t until be used.
As we reported previously, pieces of 2-mm of teeth enamel and 2-mm of dentin (n = 20) were made and a mesiodistal cut was performed using a diamond disk. After, teeth roots were removed and the dentin surface was reduced with 400 and 600 grit silicon carbide paper Sof-lex™ discs (3 M Dental Products, St Paul, MN, USA) until the dentin thickness was 2-mm. Sample sizes ranged from 0.5 cm−0.7 cm × 0.4 cm–0.6 cm. After, the dentin surface was treated with 0.5 M EDTA in PBS pH 7.4 for 30 sec, to remove the smear layer without opening dentinal tubules diameter25 and stored in bidistilled water at 4 °C until be used. Before start experimental process, samples were randomly divided in four groups (ni = 5).
As reported previously by our group, an artificial pulp chamber, with a capacity of 100 μL, was prepared with heavy silicone (Panasil R Putty, Kettenbach, Huntington Beach, CA, USA). A heavy silicone ring was fabricated to anchor the sample, allowing the dentin to be in contact with the buffer and had an upper window where to place the study gel. This ring fit perfectly in the artificial pulp chamber that maintained fixation of the sample; additional sealing was performed between the sample and the ring by using wax25.
Evaluation of diffusion
Four commercial bleaching products manufactured by two different companies were evaluated. Normon products: neutral pH gel of 37.5% content of hydrogen peroxide (HP) Norblanc Office (Nor-HP) or with 16% carbamide peroxide (CP) Norblanc Home (Nor-CP) (Laboratorios Normon, S.A., Madrid, Spain). Ultradent products: neutral pH gel of 40% HP Opalescence Boost 40% PF (Opal-HP) or 16% CP Opalescence PF 16% (Opal-CP) (Ultradent Products, Inc, South Jordan, UT, USA).
Analysis diffusion of HP from the studied bleaching compounds was carried out by fluorimetric techniques. Measurement of homovanilic acid dimer formation from a reaction catalysed by peroxidase using HP as a substrate was measured using a fluorimeter (model F-4500 fluorescence spectrophotometer, Hitachi, Japan). Previously, we made a standard fluorimetry signal curve for HP (H1009-100ML, Sigma-Aldrich). Alternatively, same dilutions were analyzed in a spectronic Helios alpha double-beam UV-Visible scanning spectrophotometer at 240 nm (Thermo Fisher Scientific Inc., Waltman, MA, USA). After, the fluorimetry data was related to the fluorescent dimer concentration.
After, a volume of 400 μL of PBS was added in the reservoir and 5 μL of the bleaching gel was applied to the external teeth enamel surface. The HP was maintained for 30 min in the Nor-HP and Opal-HP groups and for 90 min in the case of Nor-CP or Opal-CP gels. All the bleaching process was carried out at 37 °C.
Finally, a volume of 100 μL of diffused HP-containing sample was taken out and diluted with 1400 μL of the reaction buffer and diluted again with and additional volume of 500 μL glycine-EDTA buffer after 15 min. Finally, concentration values were calculated after extrapolating fluorescence intensity data into the standard curve, as reported25.
Biological assays
hDPSCs isolation and characterization
Human DPSCs (hDPSCs) were isolated from extracted third molars from healthy donors (n = 10) as reported41,42. Written informed consent was obtained from donors in accordance with Helsinki declaration guidelines and Ethics Committee of our Institution (University of Murcia; ID: 1417/2016). First, dental pulp was extracted from the pulp chamber and root canals, extensively rinsed with Hank’s balance salt solution and submitted to enzymatic digestion with 3 mg/mL collagenase A (Roche Diagnostics, Basel, Switzerland) for 1 h at 37 °C. After, the obtained cells were seeded into 75-cm2 culture flasks (Corning, New York, USA) and cultured in Alpha-MEM medium containing 100 U/mL penicillin and streptomycin and 10% fetal bovine serum (FBS) in an incubator at 37 °C and 5% CO2. Human DPSCs used for the subsequent experiments were from passage 4 onwards.
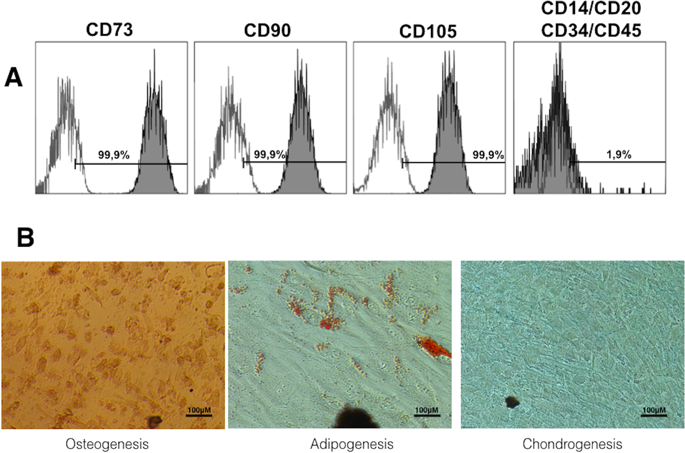
After, cultured hDPSCs was analyzed by flow cytometry using specific anti-human antibodies against CD73, CD90, CD105, CD34, CD45, CD14 and CD20 (Human MSC Phenotyping Cocktail, Miltenyi Biotec, Bergisch Gladbach, Germany), following the criteria recommended by the International Society for Cellular Therapy (ISCT)43. Also, the mesodermal differentiation potential of hDPSCs toward the adipogenic, osteogenic and chondrogenic lineages was analyzed. Adipogenic differentiation was conducted after culturing cells in StemMACS AdipoDiff media (Miltenyi Biotec) for 14 days. Then, hPDSCs were fixed with 4% paraformaldehide (PFA) and stained with Oil Red O solution (Sigma-Aldrich, St. Louis, MO, USA) to analyze formation of neutral lipid-containing cytoplasmic vacuoles. To assess osteogenic differentiation potential, hPDSCs were cultured in StemMACS OsteoDiff media (Miltenyi Biotec) for 21 days. Then, cells were fixed with 4% PFA and stained with Alizarin Red solution (Sigma-Aldrich, St. Louis, MO, USA) to analyze the formation of calcium-rich deposits in the cultures. Finally, chondrogenic differentiation was induced using StemMACS CondroDiff media (Miltenyi Biotec) for 21 days. Cells were fixed as above and analyzed with Alcian blue staining (Sigma-Aldrich) to detect mucopolysaccharides and acidic mucins.
Conditioned medium
For obtention of conditioned culture medium (CM) containing the bleaching products, we followed a protocol previously described44 and in compliance with the ISO 10993-12 procedure. In brief, 100 μL of the diffused product sample was taken out and diluted to 1%, 0.5% and 0.25% with culture medium. After, CMs were filtered and stored until be used in the different assays.
Metabolic activity assay
The cytotoxicity of the extracts to the stem cells was assessed using the MTT assay (MTT Cell Growth Kit, Chemicon, Rosemont, IL, USA), as reported previously25. Briefly, hDPSCs were seeded at 1 × 104 cells/well in a volume of 180 μL DMEM medium w/o phenol red in 96-well plates. Previously, hDPSCs were starved for 24 h in serum-free culture medium at 37 °C and subsequently exposed the different bleaching eluates. Cells cultured in Alpha-MEM medium plus 10% FBS served as positive control. A final concentration of 1 mg/mL MTT was added after 24, 48 and 72 h of culture and incubated for 4 h. Finally, the MTT-containing medium was removed, and 100 µL of dimethyl sulfoxide (DMSO) was added to solubilize formazan. Absorbance at 570 nm (Abs570) was determined, using Abs690 as the reference wavelength. Each condition was performed in triplicate.
Analysis of cell viability by flow cytometry
To analyze cell viability of hDPSCs after treatment with the different bleaching extracts for 72 h, cells were stained with Annexin-V and 7-AAD (Immunostep, Salamanca, Spain) following manufacturer’s instructions. Subsequently, percentages of live, early or late apoptotic and necrotic cells were determined in a flow cytometer.
Measurement of intracellular ROS
The production of ROS (reactive oxygen species) was analyzed using the cell-permeable general oxidative sensor 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) (Molecular Probes, Eugene, OR, USA). Briefly, hDPSCs were incubated with 5 μM CM-H2DCFDA at 37 °C for 30 min. After, intracellular ROS generation was evaluated by flow cytometry in living cells.
Immunocytochemical staining
Phalloidin staining and nuclear labeling with DAPI were performed as previously described25. For determine the effects of bleaching products on cell morphology, hDPSCs were adhered on glass coverslips in culture medium containing the different material extracts. Briefly, cells were fixed in 4% paraformaldehyde in PBS for 20 min at 37 °C, permeabilized with 0.25% Triton X-100 (Sigma-Aldrich) in PBS for 3 min, and stained with CruzFluor594-conjugated phalloidin (Santa Cruz Biotechnology, Dallas, TX, USA). Also, nuclei were labelled with 4,6-diamidino-2-phenylindole dihydrochloride (DAPI) (Sigma-Aldrich). Finally, fluorescence micrographs were acquired using a fluorescence confocal microscopy (Zeiss, Oberkochen, Germany).
Animals
Rats were supplied by the Animal Care Facility from the University of Murcia (Spain) (REGA ES300305440012). All procedures involving the use of animals were previously approved by the Bioethics Committee from the University of Murcia and the pertinent competent authority (A1320141001), and followed European Union guidelines for animal experimentation (EU/63/2010).
A total of 15 male Wistar rats with a mean weight of 350 g, were employed in the in vivo study. Rats were kept in a standard animal room at 22 ± 1 °C, 55 ± 10% humid atmosphere, 12-hour light-dark cycle, and free access to food and water ad libitum.
Five groups of n = 3 animals per group were used: i) Opal-HP, ii) Nor-HP, iii) Opal-CP, iv) Nor-CP and v) placebo gel. The bleaching gel (0.01 mL) was applied to the surface of the molars13 according to the manufacturer’s recommendations. The animals were anesthetized by intraperitoneal administration of ketamine (Ketavet 100, Gellini Farmaceutici Spa, Peschira Borromea-MI, 32 mg/kg) and xylazine (Rompun, Bayer AG, Leverkusen-Germania, 20 mg/kg).
Two days after the application of the dental bleaching treatment, animals were sacrificed, the hemi-maxillae was fixed in 4% neutral buffered formalin (Panreac Quimica, Barcelona, Spain) for 24 hours, and immersed for one week in a 10% aqueous formic acid commercial solution (TBD-2, Thermo Corp., Madrid, Spain) for bone decalcification. After, bone samples were embedded in paraffin. Three micrometers sections were then obtained for all specimens, and stained with a standard hematoxylin and eosin staining for routine histopathological examination.
To determine the degree of damage induced by the bleaching procedures in dental pulp, a previously proposed scale was used15. This semi-quantitative scale establish a score according to the relative numbers of inflammatory leukocytes per high power field (HPF) (400x), and comprised 5 degrees: grade 1 (absence of inflammatory infiltrate or negligible in number), grade 2 (mild inflammatory infiltrate, i.e. <25 cells/HPF), grade 3 (moderate inflammatory infiltrate, i.e. 25–125 cells/HPF), grade 4 (severe inflammatory infiltrate, i.e. >125 cells/HPF) and grade 5 (necrosis). All examinations were performed by using an upright light microscope (Zeiss Axio Scope A10, Zeiss, Madrid, Spain), with a digital camera (Axio Cam Icc3, Carl Zeiss, Jenna, Germany), by using a specific digital analysis software (AxioVision ver. 4.9.1, Zeiss, Jenna, Germany).
Statistical analysis
MTT assay, apoptosis and diffusion capacity results were analyzed using SPSS version 22.0 statistical software (SPSS, Inc., Chicago, IL, USA). All experiments were conducted in triplicate and repeated at least twice. Data of absorbances are shown as the mean ± standard deviation (SD). Comparisons between groups were analyzed using one-way ANOVA test followed by a Bonferroni post-test for multiple comparisons A p value < 0.05 was deemed significant (*p < 0.05, **p < 0.01, ***p < 0.001).