Production of samples
Following consent from the donors, freshly extracted permanent teeth were collected from the dental clinics of the University of Texas Health San Antonio (UTHSA). The teeth were cleaned of debris/stains and examined with a transilluminator (Microlux™ Transilluminator, AdDent Inc., Danbury, CT, USA) by 2 trained examiners (RF & BP). Teeth with MIH but free of caries or other malformations were selected and cleaned with pumice to remove the pellicle. MIH lesions were confirmed by experienced pediatric dentist (JAL). The diagnosis of the MIH lesion was established if the opacity presented as a well-defined alteration of the translucency of the enamel, was larger than 1 mm, had a color varying from creamy white to yellow to brown and was located on smooth buccal or lingual surfaces of a permanent first molar [2, 3]. These criteria differentiate MIH from other hypomineralization lesions such as enamel hypoplasia which present ill-defined margins, traumatic hypomineralization that occurs on anterior teeth, or white spot lesions that are found on plaque retentive surfaces or around orthodontic brackets [4, 10]. Using a water-cooled diamond wire saw, an MIH-bearing tooth block, measuring approximately 2 mm length × 2 mm width × 1.5 mm thickness, was produced from each hypomineralized lesion.
A total of 60 blocks were produced and randomly assigned to two categories: etched and unetched, in a ratio of 2:1 resulting in 40 etched blocks and 20 unetched blocks. The enamel surface of each block in etched category was treated with 32% phosphoric acid-etchant (Uni-Tech, BISCO, Inc., USA) for 5 s followed by rinsing with an air/water syringe for 20 s. The unetched group did not undergo any surface treatment.
Measurement of baseline mineral density of the tooth blocks
Micro-computed X-ray tomography (µ-CT) is a high resolution, non-destructive radiographic method of analysis that generates qualitative images and quantitative analysis of the mineral density (MD) in tooth samples [5, 51]. The scanning protocols were performed as described earlier [6] in a desktop scanner (Bruker SkyScan 1172, Kontich, Belgium) with a 0.5 mm aluminum filter at 60 kV, 167 mA beam intensity and a 0.35° rotation step with 1090 ms exposure time at each step. The image pixel size was 6 microns. Samples were stabilized inside the tube using spongy foams to prevent movement during scanning and allow their evaluation over time in the same position. 150 microns (0.15 mm) diameter volumes of interest (VOI), the borders of which extends from along the surface of the enamel down to a depth of 150 microns into the enamel towards the dentin, was determined. Enamel mineral density (MD) were measured in this VOI. A series of hydroxyapatite (HA) phantoms (Himed, Bethpage, NY, USA) with varying densities were also scanned under the same conditions and settings as the test blocks and were used to calibrate the enamel density measurements. The samples were reconstructed using NRecon (Bruker SkyScan, Aartselaar, Belgium) using a polynomial correction algorithm [6]. Using the density measuring program, Bruker-MicroCT CT-Analyser software, the linear attenuation coefficient (LAC, cm−1) was measured from the tomographic images of each sample and was used to determine the MD [51].
Recruitment of study subjects
This was a double-blind, randomized, crossover, single center, controlled in-situ study. The primary outcome to be examined was the percentage remineralization (i.e., percentage gain in MD) of the MIH lesions measured relative to the baseline MD of the MIH lesions. The approval (#: 20210570HU) of the UTHSA Institutional Review Board was obtained. Subjects were recruited from among the patients attending the dental clinics of the UTHSA School of Dentistry from the local San Antonio area. This in situ study was conducted in accordance with the ethical standards outlined in the 1964 Declaration of Helsinki and its later amendments, and in compliance with International Conference on Harmonization (ICH) Good Clinical Practice Guidelines. At the screening visit, each subject completed a medical/dental history and read and signed an informed consent form. Following consent, a visual oral health exam was performed by a licensed dentist (RF).
Fifteen subjects were recruited into the study (Fig. 1). The inclusion criteria were being between 18 to 60 years old, in good general health, and with no known history of allergy to personal care/consumer products (Table 1). Additionally, participants had to have a minimum of 20 natural uncrowned teeth (excluding third molars), no active unrestored cavities, and a normal salivary flow rate (stimulated and unstimulated flow of ≥0.7 mL/min and ≥0.2 mL/min, respectively) ascertained from a preliminary sialometry test [52]. They also had to give written informed consent, be available throughout entire study, and be willing to wear intra-oral appliance 24 h per day and use only assigned products for oral hygiene throughout the duration of the study.
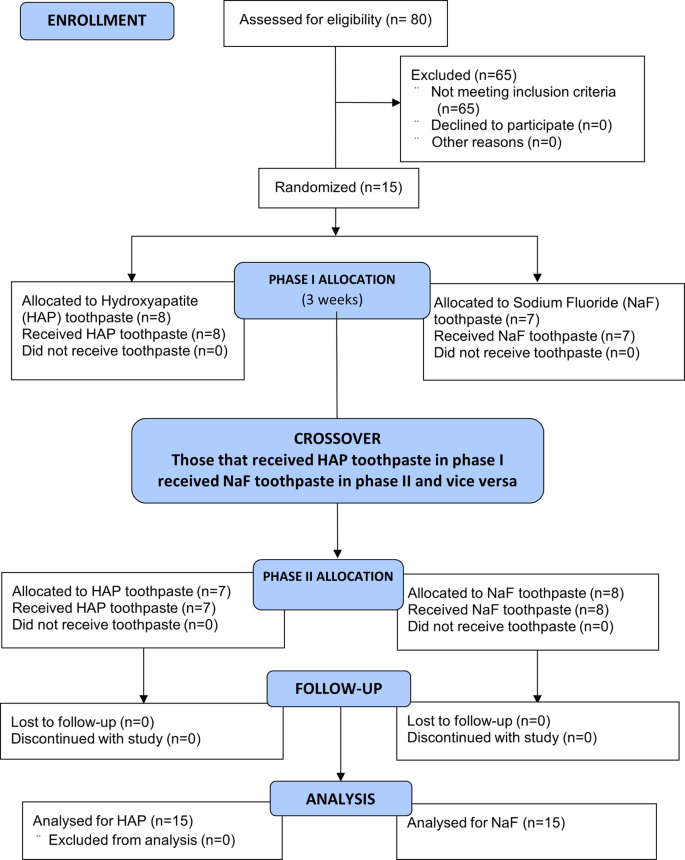
The 15 participants who completed the study, received the two interventions in a crossover design as phase I and II, each phase started with a 1-week washout period and was followed by 2-weeks of study toothpaste use.
Potential subjects were excluded if they have advanced periodontal disease, a medical condition that requires premedication prior to dental visits/procedures, an impaired salivary function, orthodontic appliances, not enough teeth to secure the intra-oral appliance, or diseases of the soft or hard oral tissues (Table 1). Other excluding criteria were using drugs that can affect salivary flow, using antibiotics one month prior to or during this study, participating in another clinical study one week prior to the start of the washout period or during this study period, using tobacco products, having history of allergy to common toothpaste ingredients, and having a compromised immune system (HIV, AIDS, immuno-suppressive drug therapy) as determined by review of medical history.
Construction of the intra-oral appliance
Following recruitment, an impression of each subject’s lower dentition was taken using an alginate impression material. A dental technician fabricated a lower removable intra-oral appliance. All tooth blocks were then covered with polyester gauze (Bard Peripheral Vascular, Inc., USA), which facilitated plaque retention on the surface of the tooth blocks on intra-oral exposure [21]. Each of the MIH-lesion-bearing blocks were mounted on each side within the acrylic portion of the removable appliance, using Intermediate Restorative Material (IRM) cement (fluoride–free). All appliances were sterilized with ethylene oxide prior to delivery to the subject.
Test product description and labeling
The toothpastes used for treatments (Table 2) were a 20% microcrystalline HAP toothpaste (test), a 1450 ppm fluoride (provided as NaF) toothpaste (comparator), and a non-fluoride, non-HAP toothpaste (washout). The test and comparator products were coded by the manufacturing/packaging company that retained the code until the completion of the study and data interpretation. The experiment consisted of two distinct treatment phases, Phase 1 and Phase 2, during which subjects were exposed to one of the two products in a randomized crossover design.
Study grouping
The 40 etched blocks were randomly assigned to two product groups: HAP or fluoride toothpastes (20 blocks/group). The blocks were assigned such that the mean MD of the two groups did not differ significantly. Similarly, the etched blocks were randomly assigned to the same HAP or fluoride groups (10 blocks/group), and again, the assignment was such that the mean MD of the two unetched groups did not differ significantly. Thus, each product group has 30 MIH-bearing blocks (20 etched and 10 unetched). The combined mean MD of the etched and unetched blocks in HAP group (n = 30) did not differ from that of the fluoride group (n = 30). Following the grouping, enrolled subjects were identified with code numbers (CP01 to CP15) and then randomly assigned to one of two treatment sequences: use of HAP toothpaste in phase 1 then NaF toothpaste in phase 2 or vice versa.
Study procedure and patient instructions
Prior to each 2-week treatment phase, subjects completed a 1-week washout period. This period allows for attenuation of any residual effect of the subject’s previously used toothpaste. During this period, no appliance was worn, and subjects used only the provided washout toothpaste and a soft-bristled toothbrush twice daily. Following the washout period, the intra-oral appliance was fitted to each subject by a qualified dentist (RF). However, before fitting the appliance, every subject received professional teeth cleaning to ensure that every subject started at the same baseline oral hygiene. Immediately after fitting of the first appliance (on day 1 of the first treatment phase), each subject received a soft bristled manual toothbrush for use throughout the duration of the study and a toothpaste according to the treatment phase. They made the first use of the test product under the supervision of the study coordinator. For the remainder of the study, subjects completed the procedure at home and as instructed.
The subjects were instructed to brush their teeth with the appliance in the mouth, two times daily, for 3 min on each brushing episode, in the morning after breakfast and last thing before bed, followed by rinsing with 10 mL of water. In dispensing the toothpaste onto the toothbrush, the subjects had to fill the toothbrush surface from end to end but no more than one ribbon of toothpaste. The subjects were advised not to brush directly on the blocks but rather to brush around the blocks to prevent disruption of the plaque. Subjects were required to neither eat nor take any drink for at least 30 min after brushing and were also instructed to remove the appliance while eating. A timer and measuring cup were provided to each subject. To monitor product usage and compliance, a diary was provided to each subject and checked at every visit, to record the number of tooth-brushings performed each day and the time it was done. Further, subjects were instructed to return the remaining toothpaste after each washout or treatment phase. The weight of toothpaste was measured before and after each treatment phase. Over the study period, all subjects had to maintain their normal dietary habits.
On day 15, the subject, without using the product that morning, arrived at the clinic, and the tooth block was harvested and sent to the laboratory for µCT analysis. Then the subject was given washout toothpaste and a soft-bristle toothbrush to undergo another 7-day washout period without an appliance. After completion of the second washout period, subjects returned to the clinic, and the appliance, with new MIH-bearing tooth blocks mounted, was fitted to the subject for the phase 2 treatment period. This procedure was then repeated until the 2-week treatment phase was completed, and each subject had gone through the two arms of the study.
Upon completion of the treatment phases, the post-remineralization mineral density (MDt) of all the MIH-lesion-bearing blocks exposed intra-orally for remineralization was assessed with µCT. This process generated the pre-test (MDb) and post-test (MDt) mineral density of the lesions and their associated images.
At every visit, the dental examiner visually examined the soft and hard tissues of the oral cavity and peri-oral area using a dental light and dental mirror. Additionally, subjects were asked about and examined for any adverse events. Subjects were able to call and request a visit for any concern of potential adverse event.
Data handling
Using the µ-CT images, the pattern and the extent of remineralization produced within each lesion by each treatment product was examined and described. This was clearly shown by comparing the pre- and post-test images side-by-side. For all calculations, the absolute MD was measured and used. The mean values of the MDb and MDt for each product group was compared using paired t-test to determine any significant change (remineralization) made by the test product (intra-group comparison). However, to make comparisons between the two products (intergroup comparison), percentage change in MD calculated relative to the baseline value was determined for each product group as follows.
% Change in MD (%ΔMD) = [(MDt – MDb)/MDb] x 100.
Sample size calculation
The power analysis and sample size calculation were performed using nQuery Advisor software (Statistical Solutions, Cork, Ireland). The sample size for this study was based on the primary efficacy outcome, %ΔMD, following 2 weeks of treatment. A sufficient number of subjects were screened to randomize 15 subjects with the intention that approximately 15 subjects complete all study treatments and be evaluable for the efficacy analysis. With these conditions met, the study has 80% power at the 5% significance level, using two-sided testing, to detect a mean treatment difference in %ΔMD of approximately 8.1% assuming a within subject standard deviation of approximately 12.6%. In the absence of available 2-week data from previous mineral density studies, the within subject standard deviation has been estimated using 14-day post-treatment data from previous results obtained by this research group in a demineralization and remineralization study using HAP toothpaste [21]. In that study the mean % remineralization was equal to 30.3 with a standard deviation equal to 16.3.
Statistical analysis
All analyses were performed using SPSS 28. The data was analyzed at three levels: all data combined, etched data only, and unetched data only. Paired samples t-test, comparing the mean values of the pre-treatment and the post-treatment MD for each product (Intra-group comparison), was used to address the first null hypothesis that none of the two toothpaste formulations would promote remineralization of hypomineralized enamel that is significantly greater than zero. The statistical significance for each analysis was established at an alpha level of α = 0.05. The mean percentage remineralization was determined for each product group as a percentage of pre-treatment MD. Using their mean percentage remineralization, the two product groups were compared (inter-group comparison) by independent group t-test, to address the second hypothesis that there would not be a practical significant difference between the efficacy of the two products in remineralizing MIH lesions. As stated earlier in the ‘Introduction’ section, in this comparison we applied the concept of practical significance, which adapted to deal with cases where groups have different standard deviations. In the present study, a practical significance was established if the difference in the mean percentage remineralization between the two toothpaste formulations is ≥10% with a Cohen’s d large effect size.
Preliminary analyses were conducted to explore the dataset and to check for assumptions violations. Specific t-test assumptions tests including tests for independence, normality and extreme outliers were carried out. The assumptions of independence and that of normality were met. The normality assumption was tested using the histogram, Q-Q plot and the Shapiro-Wilk’s test from the tests of normality table and all confirmed that the normality assumption was met for each variable at the alpha level of α = 0.05.

