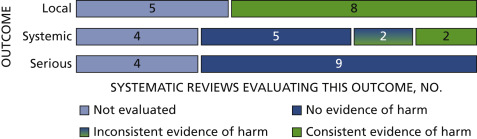
In all but 6 of the systematic reviews,37x37Harder, T., Wichmann, O., Klug, S.J., van der Sande, M.A.B., and Wiese-Posselt, M. Efficacy, effectiveness and safety of vaccination against human papillomavirus in males: a systematic review. BMC Med. 2018;
16: 110–127
Google ScholarSee all References,40x40Luo, W., Zhang, S.H., Zhou, Y.Z. et al. Safety and immunogenicity of quadrivalent HPV vaccine: a meta-analysis. Chin J Evid Based Med. 2015;
15: 47–53
Google ScholarSee all References,51x51Sangar, V.C., Ghongane, B.B., Mathur, G., and Chowdhary, A.S. Safety and adverse events of prophylactic HPV vaccines among healthy women: a systematic review & meta analysis. Int J Pharm Sci Res. 2015;
6: 1779–1791
Google ScholarSee all References,53x53Signorelli, C., Odone, A., Ciorba, V. et al. Human papillomavirus 9-valent vaccine for cancer prevention: a systematic review of the available evidence. Epidemiol Infect. 2017;
145: 1962–1982
Google ScholarSee all References,56x56Yakely, A.E., Avni-Singer, L., Oliveira, C.R., and Niccolai, L.M. Human papillomavirus vaccination and anogenital warts: a systematic review of impact and effectiveness in the United States. Sex Transm Dis. 2018;
46: 213–220
Google ScholarSee all References,57x57Yancey, A.M., Pitlick, J.M., and Forinash, A.B. The prophylactic role for the human papillomavirus quadrivalent vaccine in males. Annal Pharmacother. 2010;
44: 1314–1318
Google ScholarSee all References researchers reported on outcomes when administering more than 1 HPV vaccine. In 7 studies, researchers reported outcomes after administration of the monovalent HPV vaccine,30x30Arbyn, M., Xu, L., Simoens, C., and Martin-Hirsch, P.P.L. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;
2018
Google ScholarSee all References,32x32Couto, E., Sæterdal, I., Juvet, L.K., and Klemp, M. HPV catch-up vaccination of young women: a systematic review and meta-analysis. BMC Public Health. 2014;
14 ()
Google ScholarSee all References,38x38La Torre, G., de Waure, C., Chiaradia, G., Mannocci, A., and Ricciardi, W. HPV vaccine efficacy in preventing persistent cervical HPV infection: a systematic review and meta-analysis. Vaccine. 2007;
25: 8352–8358
Google ScholarSee all References,39x39Lu, B., Kumar, A., Castellsagué, X., and Giuliano, A.R. Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review & meta-analysis. BMC Infect Dis. 2011;
11 ()
Google ScholarSee all References,47x47Miltz, A., Price, H., Shahmanesh, M., Copas, A., and Gilson, R. Systematic review and meta-analysis of L1-VLP-based human papillomavirus vaccine efficacy against anogenital pre-cancer in women with evidence of prior HPV exposure. PLoS One. 2014;
9
Google ScholarSee all References,49x49Rambout, L., Hopkins, L., Hutton, B., and Fergusson, D. Prophylactic vaccination against human papillomavirus infection and disease in women: a systematic review of randomized controlled trials. CMAJ. 2007;
177: 469–479
Google ScholarSee all References,54x54Tan, P., Wang, X., Wei, S. et al. Efficacy and safety of prophylactic human papillomavirus vaccination in healthy males: a meta-analysis. Rev Med Microbiol. 2015;
26: 143–153
Google ScholarSee all References in 25 after administration of the bivalent HPV vaccine,28x28Agorastos, T., Chatzigeorgiou, K., Brotherton, J.M., and Garland, S.M. Safety of human papillomavirus (HPV) vaccines: a review of the international experience so far. Vaccine. 2009;
27: 7270–7281
Google ScholarSee all References,29x29Araujo, S.C., Caetano, R., Braga, J.U., and Costa e Silva, F.V. Efficacy of commercially available vaccines against HPV infection in women: a systematic review and meta-analysis [in Portuguese]. Cad Saude Publica. 2013;
29: S32–S44
Google ScholarSee all References,30x30Arbyn, M., Xu, L., Simoens, C., and Martin-Hirsch, P.P.L. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;
2018
Google ScholarSee all References,31x31Coelho, P.L., da Silva Calestini, G.L., Alvo, F.S., de Moura Freitas, J.M., Castro, P.M., and Konstantyner, T. Safety of human papillomavirus 6, 11, 16 and 18 (recombinant): wystematic review and meta-analysis [in Portuguese]. Rev Paul Pediatr. 2015;
33: 474–482
Google ScholarSee all References,32x32Couto, E., Sæterdal, I., Juvet, L.K., and Klemp, M. HPV catch-up vaccination of young women: a systematic review and meta-analysis. BMC Public Health. 2014;
14 ()
Google ScholarSee all References,33x33Deleré, Y., Wichmann, O., Klug, S.J. et al. The efficacy and duration of vaccine protection against human papillomavirus. Dtsch Arztebl Int. 2014;
111: 584–591 ()
Google ScholarSee all References,34x34Di Mario, S., Basevi, V., Lopalco, P.L., Balduzzi, S., D’Amico, R., and Magrini, N. Are the two human papillomavirus vaccines really similar? A systematic review of available evidence: efficacy of the two vaccines against HPV. J Immunol Res. 2015;
2015: 13
Google ScholarSee all References,35x35Garland, S.M., Kjaer, S.K., Muñoz, N. et al. Impact and effectiveness of the quadrivalent human papillomavirus vaccine: a systematic review of 10 years of real-world experience. Clin Infect Dis. 2016;
63: 519–527
Google ScholarSee all References,36x36Goncalves, A.K., Cobucci, R.N., Rodrigues, H.M., de Melo, A.G., and Giraldo, P.C. Safety, tolerability and side effects of human papillomavirus vaccines: a systematic quantitative review. Braz J Infect Dis. 2014;
18: 651–659
Google ScholarSee all References,38x38La Torre, G., de Waure, C., Chiaradia, G., Mannocci, A., and Ricciardi, W. HPV vaccine efficacy in preventing persistent cervical HPV infection: a systematic review and meta-analysis. Vaccine. 2007;
25: 8352–8358
Google ScholarSee all References,39x39Lu, B., Kumar, A., Castellsagué, X., and Giuliano, A.R. Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review & meta-analysis. BMC Infect Dis. 2011;
11 ()
Google ScholarSee all References,41x41Macartney, K.K., Chiu, C., Georgousakis, M., and Brotherton, J.M.L. Safety of human papillomavirus vaccines: a review. Drug Saf. 2013;
36: 393–412
Google ScholarSee all References,42x42Macki, M. and Dabaja, A.A. Literature review of vaccine-related adverse events reported from HPV vaccination in randomized controlled trials. Basic Clin Androl. 2016;
26: 16
Google ScholarSee all References,43x43Malagón, T., Drolet, M., Boily, M.C. et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;
12: 781–789
Google ScholarSee all References,44x44Markowitz, L.E., Drolet, M., Perez, N., Jit, M., and Brisson, M. Human papillomavirus vaccine effectiveness by number of doses: systematic review of data from national immunization programs. Vaccine. 2018;
36: 4806–4815
Google ScholarSee all References,45x45Martínez-Lavín, M. and Amezcua-Guerra, L. Serious adverse events after HPV vaccination: a critical review of randomized trials and post-marketing case series. Clin Rheumatol. 2017;
36: 2169–2178
Google ScholarSee all References,46x46Medeiros, L.R., Rosa, D.D., da Rosa, M.I., Bozzetti, M.C., and Zanini, R.R. Efficacy of human papillomavirus vaccines: a systematic quantitative review. Int J Gynecol Cancer. 2009;
19: 1166–1176
Google ScholarSee all References,47x47Miltz, A., Price, H., Shahmanesh, M., Copas, A., and Gilson, R. Systematic review and meta-analysis of L1-VLP-based human papillomavirus vaccine efficacy against anogenital pre-cancer in women with evidence of prior HPV exposure. PLoS One. 2014;
9
Google ScholarSee all References,48x48Ogawa, Y., Takei, H., Ogawa, R., and Mihara, K. Safety of human papillomavirus vaccines in healthy young women: a meta-analysis of 24 controlled studies. J Pharm Health Care Sci. 2017;
3
Google ScholarSee all References,49x49Rambout, L., Hopkins, L., Hutton, B., and Fergusson, D. Prophylactic vaccination against human papillomavirus infection and disease in women: a systematic review of randomized controlled trials. CMAJ. 2007;
177: 469–479
Google ScholarSee all References,50x50Rey-Ares, L., Ciapponi, A., and Pichon-Riviere, A. Efficacy and safety of human papilloma virus vaccine in cervical cancer prevention: systematic review and meta-analysis. Arch Argent Pediatr. 2012;
110: 483–489
Google ScholarSee all References,51x51Sangar, V.C., Ghongane, B.B., Mathur, G., and Chowdhary, A.S. Safety and adverse events of prophylactic HPV vaccines among healthy women: a systematic review & meta analysis. Int J Pharm Sci Res. 2015;
6: 1779–1791
Google ScholarSee all References,52x52Setiawan, D., Luttjeboer, J., Pouwels, K.B., Wilschut, J.C., and Postma, M.J. Immunogenicity and safety of human papillomavirus (HPV) vaccination in Asian populations from six countries: a meta-analysis. Jpn J Clin Oncol. 2017;
47: 265–276
Google ScholarSee all References,54x54Tan, P., Wang, X., Wei, S. et al. Efficacy and safety of prophylactic human papillomavirus vaccination in healthy males: a meta-analysis. Rev Med Microbiol. 2015;
26: 143–153
Google ScholarSee all References,55x55Tejada, R.A., Vargas, K.G., Benites-Zapata, V., Mezones-Holguín, E., Bolaños-Díaz, R., and Hernandez, A.V. Human papillomavirus vaccine efficacy in the prevention of anogenital warts: systematic review and meta-analysis. Salud Publica Mex. 2017;
59: 84–94
Google ScholarSee all References in 28 after administration of the quadrivalent HPV vaccine,28x28Agorastos, T., Chatzigeorgiou, K., Brotherton, J.M., and Garland, S.M. Safety of human papillomavirus (HPV) vaccines: a review of the international experience so far. Vaccine. 2009;
27: 7270–7281
Google ScholarSee all References,29x29Araujo, S.C., Caetano, R., Braga, J.U., and Costa e Silva, F.V. Efficacy of commercially available vaccines against HPV infection in women: a systematic review and meta-analysis [in Portuguese]. Cad Saude Publica. 2013;
29: S32–S44
Google ScholarSee all References,30x30Arbyn, M., Xu, L., Simoens, C., and Martin-Hirsch, P.P.L. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;
2018
Google ScholarSee all References,31x31Coelho, P.L., da Silva Calestini, G.L., Alvo, F.S., de Moura Freitas, J.M., Castro, P.M., and Konstantyner, T. Safety of human papillomavirus 6, 11, 16 and 18 (recombinant): wystematic review and meta-analysis [in Portuguese]. Rev Paul Pediatr. 2015;
33: 474–482
Google ScholarSee all References,32x32Couto, E., Sæterdal, I., Juvet, L.K., and Klemp, M. HPV catch-up vaccination of young women: a systematic review and meta-analysis. BMC Public Health. 2014;
14 ()
Google ScholarSee all References,33x33Deleré, Y., Wichmann, O., Klug, S.J. et al. The efficacy and duration of vaccine protection against human papillomavirus. Dtsch Arztebl Int. 2014;
111: 584–591 ()
Google ScholarSee all References,34x34Di Mario, S., Basevi, V., Lopalco, P.L., Balduzzi, S., D’Amico, R., and Magrini, N. Are the two human papillomavirus vaccines really similar? A systematic review of available evidence: efficacy of the two vaccines against HPV. J Immunol Res. 2015;
2015: 13
Google ScholarSee all References,35x35Garland, S.M., Kjaer, S.K., Muñoz, N. et al. Impact and effectiveness of the quadrivalent human papillomavirus vaccine: a systematic review of 10 years of real-world experience. Clin Infect Dis. 2016;
63: 519–527
Google ScholarSee all References,36x36Goncalves, A.K., Cobucci, R.N., Rodrigues, H.M., de Melo, A.G., and Giraldo, P.C. Safety, tolerability and side effects of human papillomavirus vaccines: a systematic quantitative review. Braz J Infect Dis. 2014;
18: 651–659
Google ScholarSee all References,37x37Harder, T., Wichmann, O., Klug, S.J., van der Sande, M.A.B., and Wiese-Posselt, M. Efficacy, effectiveness and safety of vaccination against human papillomavirus in males: a systematic review. BMC Med. 2018;
16: 110–127
Google ScholarSee all References,38x38La Torre, G., de Waure, C., Chiaradia, G., Mannocci, A., and Ricciardi, W. HPV vaccine efficacy in preventing persistent cervical HPV infection: a systematic review and meta-analysis. Vaccine. 2007;
25: 8352–8358
Google ScholarSee all References,39x39Lu, B., Kumar, A., Castellsagué, X., and Giuliano, A.R. Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review & meta-analysis. BMC Infect Dis. 2011;
11 ()
Google ScholarSee all References,40x40Luo, W., Zhang, S.H., Zhou, Y.Z. et al. Safety and immunogenicity of quadrivalent HPV vaccine: a meta-analysis. Chin J Evid Based Med. 2015;
15: 47–53
Google ScholarSee all References,41x41Macartney, K.K., Chiu, C., Georgousakis, M., and Brotherton, J.M.L. Safety of human papillomavirus vaccines: a review. Drug Saf. 2013;
36: 393–412
Google ScholarSee all References,42x42Macki, M. and Dabaja, A.A. Literature review of vaccine-related adverse events reported from HPV vaccination in randomized controlled trials. Basic Clin Androl. 2016;
26: 16
Google ScholarSee all References,43x43Malagón, T., Drolet, M., Boily, M.C. et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;
12: 781–789
Google ScholarSee all References,44x44Markowitz, L.E., Drolet, M., Perez, N., Jit, M., and Brisson, M. Human papillomavirus vaccine effectiveness by number of doses: systematic review of data from national immunization programs. Vaccine. 2018;
36: 4806–4815
Google ScholarSee all References,45x45Martínez-Lavín, M. and Amezcua-Guerra, L. Serious adverse events after HPV vaccination: a critical review of randomized trials and post-marketing case series. Clin Rheumatol. 2017;
36: 2169–2178
Google ScholarSee all References,46x46Medeiros, L.R., Rosa, D.D., da Rosa, M.I., Bozzetti, M.C., and Zanini, R.R. Efficacy of human papillomavirus vaccines: a systematic quantitative review. Int J Gynecol Cancer. 2009;
19: 1166–1176
Google ScholarSee all References,47x47Miltz, A., Price, H., Shahmanesh, M., Copas, A., and Gilson, R. Systematic review and meta-analysis of L1-VLP-based human papillomavirus vaccine efficacy against anogenital pre-cancer in women with evidence of prior HPV exposure. PLoS One. 2014;
9
Google ScholarSee all References,48x48Ogawa, Y., Takei, H., Ogawa, R., and Mihara, K. Safety of human papillomavirus vaccines in healthy young women: a meta-analysis of 24 controlled studies. J Pharm Health Care Sci. 2017;
3
Google ScholarSee all References,49x49Rambout, L., Hopkins, L., Hutton, B., and Fergusson, D. Prophylactic vaccination against human papillomavirus infection and disease in women: a systematic review of randomized controlled trials. CMAJ. 2007;
177: 469–479
Google ScholarSee all References,50x50Rey-Ares, L., Ciapponi, A., and Pichon-Riviere, A. Efficacy and safety of human papilloma virus vaccine in cervical cancer prevention: systematic review and meta-analysis. Arch Argent Pediatr. 2012;
110: 483–489
Google ScholarSee all References,52x52Setiawan, D., Luttjeboer, J., Pouwels, K.B., Wilschut, J.C., and Postma, M.J. Immunogenicity and safety of human papillomavirus (HPV) vaccination in Asian populations from six countries: a meta-analysis. Jpn J Clin Oncol. 2017;
47: 265–276
Google ScholarSee all References,54x54Tan, P., Wang, X., Wei, S. et al. Efficacy and safety of prophylactic human papillomavirus vaccination in healthy males: a meta-analysis. Rev Med Microbiol. 2015;
26: 143–153
Google ScholarSee all References,55x55Tejada, R.A., Vargas, K.G., Benites-Zapata, V., Mezones-Holguín, E., Bolaños-Díaz, R., and Hernandez, A.V. Human papillomavirus vaccine efficacy in the prevention of anogenital warts: systematic review and meta-analysis. Salud Publica Mex. 2017;
59: 84–94
Google ScholarSee all References,56x56Yakely, A.E., Avni-Singer, L., Oliveira, C.R., and Niccolai, L.M. Human papillomavirus vaccination and anogenital warts: a systematic review of impact and effectiveness in the United States. Sex Transm Dis. 2018;
46: 213–220
Google ScholarSee all References,57x57Yancey, A.M., Pitlick, J.M., and Forinash, A.B. The prophylactic role for the human papillomavirus quadrivalent vaccine in males. Annal Pharmacother. 2010;
44: 1314–1318
Google ScholarSee all References and in 3 after administration of the nonavalent HPV vaccine.45x45Martínez-Lavín, M. and Amezcua-Guerra, L. Serious adverse events after HPV vaccination: a critical review of randomized trials and post-marketing case series. Clin Rheumatol. 2017;
36: 2169–2178
Google ScholarSee all References,48x48Ogawa, Y., Takei, H., Ogawa, R., and Mihara, K. Safety of human papillomavirus vaccines in healthy young women: a meta-analysis of 24 controlled studies. J Pharm Health Care Sci. 2017;
3
Google ScholarSee all References,53x53Signorelli, C., Odone, A., Ciorba, V. et al. Human papillomavirus 9-valent vaccine for cancer prevention: a systematic review of the available evidence. Epidemiol Infect. 2017;
145: 1962–1982
Google ScholarSee all References Researchers in 13 reviews did not indicate whether the results presented were based on intention-to-treat or per-protocol analysis.28x28Agorastos, T., Chatzigeorgiou, K., Brotherton, J.M., and Garland, S.M. Safety of human papillomavirus (HPV) vaccines: a review of the international experience so far. Vaccine. 2009;
27: 7270–7281
Google ScholarSee all References,31x31Coelho, P.L., da Silva Calestini, G.L., Alvo, F.S., de Moura Freitas, J.M., Castro, P.M., and Konstantyner, T. Safety of human papillomavirus 6, 11, 16 and 18 (recombinant): wystematic review and meta-analysis [in Portuguese]. Rev Paul Pediatr. 2015;
33: 474–482
Google ScholarSee all References,35x35Garland, S.M., Kjaer, S.K., Muñoz, N. et al. Impact and effectiveness of the quadrivalent human papillomavirus vaccine: a systematic review of 10 years of real-world experience. Clin Infect Dis. 2016;
63: 519–527
Google ScholarSee all References,36x36Goncalves, A.K., Cobucci, R.N., Rodrigues, H.M., de Melo, A.G., and Giraldo, P.C. Safety, tolerability and side effects of human papillomavirus vaccines: a systematic quantitative review. Braz J Infect Dis. 2014;
18: 651–659
Google ScholarSee all References,40x40Luo, W., Zhang, S.H., Zhou, Y.Z. et al. Safety and immunogenicity of quadrivalent HPV vaccine: a meta-analysis. Chin J Evid Based Med. 2015;
15: 47–53
Google ScholarSee all References,41x41Macartney, K.K., Chiu, C., Georgousakis, M., and Brotherton, J.M.L. Safety of human papillomavirus vaccines: a review. Drug Saf. 2013;
36: 393–412
Google ScholarSee all References,42x42Macki, M. and Dabaja, A.A. Literature review of vaccine-related adverse events reported from HPV vaccination in randomized controlled trials. Basic Clin Androl. 2016;
26: 16
Google ScholarSee all References,44x44Markowitz, L.E., Drolet, M., Perez, N., Jit, M., and Brisson, M. Human papillomavirus vaccine effectiveness by number of doses: systematic review of data from national immunization programs. Vaccine. 2018;
36: 4806–4815
Google ScholarSee all References,45x45Martínez-Lavín, M. and Amezcua-Guerra, L. Serious adverse events after HPV vaccination: a critical review of randomized trials and post-marketing case series. Clin Rheumatol. 2017;
36: 2169–2178
Google ScholarSee all References,47x47Miltz, A., Price, H., Shahmanesh, M., Copas, A., and Gilson, R. Systematic review and meta-analysis of L1-VLP-based human papillomavirus vaccine efficacy against anogenital pre-cancer in women with evidence of prior HPV exposure. PLoS One. 2014;
9
Google ScholarSee all References,48x48Ogawa, Y., Takei, H., Ogawa, R., and Mihara, K. Safety of human papillomavirus vaccines in healthy young women: a meta-analysis of 24 controlled studies. J Pharm Health Care Sci. 2017;
3
Google ScholarSee all References,56x56Yakely, A.E., Avni-Singer, L., Oliveira, C.R., and Niccolai, L.M. Human papillomavirus vaccination and anogenital warts: a systematic review of impact and effectiveness in the United States. Sex Transm Dis. 2018;
46: 213–220
Google ScholarSee all References,57x57Yancey, A.M., Pitlick, J.M., and Forinash, A.B. The prophylactic role for the human papillomavirus quadrivalent vaccine in males. Annal Pharmacother. 2010;
44: 1314–1318
Google ScholarSee all References In 4 reviews researchers summarized and reported intention-to-treat analysis only,34x34Di Mario, S., Basevi, V., Lopalco, P.L., Balduzzi, S., D’Amico, R., and Magrini, N. Are the two human papillomavirus vaccines really similar? A systematic review of available evidence: efficacy of the two vaccines against HPV. J Immunol Res. 2015;
2015: 13
Google ScholarSee all References,37x37Harder, T., Wichmann, O., Klug, S.J., van der Sande, M.A.B., and Wiese-Posselt, M. Efficacy, effectiveness and safety of vaccination against human papillomavirus in males: a systematic review. BMC Med. 2018;
16: 110–127
Google ScholarSee all References,46x46Medeiros, L.R., Rosa, D.D., da Rosa, M.I., Bozzetti, M.C., and Zanini, R.R. Efficacy of human papillomavirus vaccines: a systematic quantitative review. Int J Gynecol Cancer. 2009;
19: 1166–1176
Google ScholarSee all References,50x50Rey-Ares, L., Ciapponi, A., and Pichon-Riviere, A. Efficacy and safety of human papilloma virus vaccine in cervical cancer prevention: systematic review and meta-analysis. Arch Argent Pediatr. 2012;
110: 483–489
Google ScholarSee all References in 3 reviews researchers presented per-protocol analysis only,38x38La Torre, G., de Waure, C., Chiaradia, G., Mannocci, A., and Ricciardi, W. HPV vaccine efficacy in preventing persistent cervical HPV infection: a systematic review and meta-analysis. Vaccine. 2007;
25: 8352–8358
Google ScholarSee all References,51x51Sangar, V.C., Ghongane, B.B., Mathur, G., and Chowdhary, A.S. Safety and adverse events of prophylactic HPV vaccines among healthy women: a systematic review & meta analysis. Int J Pharm Sci Res. 2015;
6: 1779–1791
Google ScholarSee all References,54x54Tan, P., Wang, X., Wei, S. et al. Efficacy and safety of prophylactic human papillomavirus vaccination in healthy males: a meta-analysis. Rev Med Microbiol. 2015;
26: 143–153
Google ScholarSee all References and in 10 reviews researchers presented both intention-to-treat and per-protocol analyses .29x29Araujo, S.C., Caetano, R., Braga, J.U., and Costa e Silva, F.V. Efficacy of commercially available vaccines against HPV infection in women: a systematic review and meta-analysis [in Portuguese]. Cad Saude Publica. 2013;
29: S32–S44
Google ScholarSee all References,30x30Arbyn, M., Xu, L., Simoens, C., and Martin-Hirsch, P.P.L. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;
2018
Google ScholarSee all References,32x32Couto, E., Sæterdal, I., Juvet, L.K., and Klemp, M. HPV catch-up vaccination of young women: a systematic review and meta-analysis. BMC Public Health. 2014;
14 ()
Google ScholarSee all References,33x33Deleré, Y., Wichmann, O., Klug, S.J. et al. The efficacy and duration of vaccine protection against human papillomavirus. Dtsch Arztebl Int. 2014;
111: 584–591 ()
Google ScholarSee all References,39x39Lu, B., Kumar, A., Castellsagué, X., and Giuliano, A.R. Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review & meta-analysis. BMC Infect Dis. 2011;
11 ()
Google ScholarSee all References,43x43Malagón, T., Drolet, M., Boily, M.C. et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;
12: 781–789
Google ScholarSee all References,49x49Rambout, L., Hopkins, L., Hutton, B., and Fergusson, D. Prophylactic vaccination against human papillomavirus infection and disease in women: a systematic review of randomized controlled trials. CMAJ. 2007;
177: 469–479
Google ScholarSee all References,52x52Setiawan, D., Luttjeboer, J., Pouwels, K.B., Wilschut, J.C., and Postma, M.J. Immunogenicity and safety of human papillomavirus (HPV) vaccination in Asian populations from six countries: a meta-analysis. Jpn J Clin Oncol. 2017;
47: 265–276
Google ScholarSee all References,53x53Signorelli, C., Odone, A., Ciorba, V. et al. Human papillomavirus 9-valent vaccine for cancer prevention: a systematic review of the available evidence. Epidemiol Infect. 2017;
145: 1962–1982
Google ScholarSee all References,55x55Tejada, R.A., Vargas, K.G., Benites-Zapata, V., Mezones-Holguín, E., Bolaños-Díaz, R., and Hernandez, A.V. Human papillomavirus vaccine efficacy in the prevention of anogenital warts: systematic review and meta-analysis. Salud Publica Mex. 2017;
59: 84–94
Google ScholarSee all References
| PubMed |
| “Papillomavirus Vaccines”[Mesh] OR ((“Papillomavirus Infections”[Mesh] OR “Papillomaviridae”[Mesh] OR HPV OR hrHPV† OR HPV16/18 OR HPV6 OR HPV16 OR HPV18 OR “Human papillomavirus” OR “Human papillomaviruses” OR “Human papilloma virus” OR “Human papilloma viruses” OR Gardasil OR Cervarix OR 4vHPV‡ OR 9vHPV) AND (“Vaccination”[Mesh] OR Vaccine OR Vaccines OR Vaccinate OR Vaccinated OR Vaccination OR immunize OR Immunization OR Immunizations OR immunized)) Database supplied limits: 2006 onward; Systematic reviews Filters: none |
| Embase | |
| #43 | #9 AND #41 AND [2006-2018]/py‖ |
| #42 | #9 AND #41 |
| #41 | #40 NOT #39 |
| #40 | #13 OR #22 OR #28 OR #33 |
| #39 | #34 OR #35 OR #38 |
| #38 | #36 NOT (#36 AND #37) |
| #37 | ‘human’/exp |
| #36 | ‘animal’/exp |
| #35 | ‘editorial’:it# |
| #34 | ‘letter’:it |
| #33 | #31 AND #32 |
| #32 | ‘review’:it |
| #31 | #29 OR #30 |
| #30 | ‘selection criteria’:ab |
| #29 | ‘data extraction’:ab |
| #28 | #23 OR #24 OR #25 OR #26 OR #27 |
| #27 | ‘relevant journals’:ab |
| #26 | ‘manual search*’:ab |
| #25 | ‘hand-search*’:ab |
| #24 | ‘bibliograph*’:ab |
| #23 | ‘reference lists’:ab |
| #22 | #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 |
| #21 | ‘bids’:ab |
| #20 | ‘science citation index’:ab |
| #19 | (‘cinahl’:ab OR ‘cinhal’:ab) |
| #18 | (‘psychinfo’:ab OR ‘psycinfo’:ab) |
| #17 | (‘psychlit’:ab OR ‘psyclit’:ab) |
| #16 | ’embase’:ab |
| #15 | ‘cochrane’:ab |
| #14 | ‘cancerlit’:ab |
| #13 | #10 OR #11 OR #12 |
| #12 | systematic NEXT/1 (review* OR overview*) |
| #11 | (meta NEXT/1 analy*) OR metaanalys* |
| #10 | ‘meta analysis’/exp |
| #9 | #1 OR #8 |
| #8 | #6 AND #7 |
| #7 | #4 OR #5 |
| #6 | #2 OR #3 |
| #5 | vaccine OR vaccines OR vaccinate OR vaccinated OR vaccination OR immunize OR immunization OR immunizations OR immunized |
| #4 | ‘vaccination’/exp |
| #3 | hpv OR hrhpv OR hpv1618 OR hpv6 OR hpv16 OR hpv18 OR ‘human papillomavirus’ OR ‘human papillomaviruses’ OR ‘human papilloma virus’ OR ‘human papilloma viruses’ OR gardasil OR cervarix OR 4vhpv OR 9vhpv |
| #2 | ‘papillomavirus infection’/exp OR ‘papillomaviridae’/exp |
| #1 | ‘wart virus vaccine’/exp |
| Database supplied limits: 2006 onward | |
| Filters: SIGN†† Systematic reviews filter incorporated into search strategy | |
| CUMULATIVE INDEX TO NURSING AND ALLIED HEALTH LITERATURE | |
| S1 | (MH‡‡ “Papillomavirus Vaccine”) |
| S2 | (MH “Papillomavirus Infections+”) |
| S3 | HPV OR hrHPV OR HPV16/18 OR HPV6 OR HPV16 OR HPV18 OR “Human papillomavirus” OR “Human papillomaviruses” OR “Human papilloma virus” OR “Human papilloma viruses” OR Gardasil OR Cervarix OR 4vHPV OR 9vHPV |
| S4 | S2 OR S3 |
| S5 | (MH “Immunization+”) |
| S6 | Vaccine OR Vaccines OR Vaccinate OR Vaccinated OR Vaccination OR immunize OR Immunization OR Immunizations OR immunized |
| S7 | S5 OR S6 |
| S8 | S4 AND S7 |
| S9 | S1 OR S8 |
| S10 | (MH “Meta Analysis”) |
| S11 | TX Meta analys* |
| S12 | TX Metaanaly* |
| S13 | (MH “Literature Review+”) |
| S14 | TX systematic N1 (review or overview) |
| S15 | S10 OR S11 OR S12 OR S13 OR S14 |
| S16 | PT commentary |
| S17 | PT letter |
| S18 | PT editorial |
| S19 | (MH “Animals”) |
| S20 | S16 OR S17 OR S18 OR S19 |
| S21 | S15 NOT S20 |
| S22 | S9 AND S21 |
| Database supplied limits: 2006 onward | |
| Filters: SIGN Systematic reviews filter incorporated into search strategy | |
| Cochrane Database of Systematic Reviews | |
| #1 | MeSH## descriptor: [Papillomavirus Vaccines] explode all trees |
| #2 | MeSH descriptor: [Papillomavirus Infections] explode all trees |
| #3 | MeSH descriptor: [Papillomaviridae] explode all trees |
| #4 | HPV OR hrHPV OR HPV16/18 OR HPV6 OR HPV16 OR HPV18 OR “Human papillomavirus” OR “Human papillomaviruses” OR “Human papilloma virus” OR “Human papilloma viruses” OR Gardasil OR Cervarix OR 4vHPV OR 9vHPV |
| #5 | #2 OR #3 OR #4 |
| #6 | MeSH descriptor: [Vaccination] explode all trees |
| #7 | Vaccine OR Vaccines OR Vaccinate OR Vaccinated OR Vaccination OR immunize OR Immunization OR Immunizations OR immunized |
| #8 | #6 OR #7 |
| #9 | #5 AND #8 |
| #10 | #1 OR #9 |
| Database supplied limits: 2006 onward | |
| Filters: none | |
| Prospective Register for Systematic Reviews |
| ((“human papillomavirus” OR “human papilloma virus” OR HPV) AND (vaccin* OR immun*)) Database supplied limits: none (limited by date within EndNote) Filters: none |
| Database of Abstracts of Reviews of Effects |
| HPV vaccine OR Papillomavirus Vaccines Database supplied limits: Limited to items from CRD (CRD assessed review bibliographic and full abstract) (limited by date within EndNote) Filters: none |
| PRIMARY AUTHOR, DATE | ARTICLE TITLE | REASON FOR EXCLUSION |
|---|---|---|
| Ault, 2006 | Vaccines for the prevention of human papillomavirus and associated gynecologic diseases: a review | Not a systematic review |
| Damm, 2009 | Human papillomavirus (HPV) vaccination for the prevention of HPV 16/18 induced cervical cancer and its precursors | Abstract of presentation only |
| Jeurissen, 2009 | Epidemiological and economic impact of human papillomavirus vaccines | Wrong outcomes |
| Marra, 2009 | Effectiveness and cost effectiveness of human papillomavirus vaccine: a systematic review | Wrong outcomes |
| Mougin, 2009 | [HPV immunization for the prevention of cervical cancer] | Not a systematic review |
| La Torre, 2010 | The health technology assessment of bivalent HPV vaccine Cervarix in Italy | Not a systematic review |
| McCormack, 2010 | Quadrivalent human papillomavirus (types 6,11,16,18) recombinant vaccine (Gardasil®): a review of its use in the prevention of premalignant genital lesions, genital cancer and genital warts in women | Not a systematic review |
| Pomfret, 2011 | Quadrivalent human papillomavirus (HPV) vaccine: a review of safety, efficacy, and pharmacoeconomics | Not a systematic review |
| Romanowski, 2011 | Long term protection against cervical infection with the human papillomavirus: review of currently available vaccines | Not a systematic review |
| Riaz, 2012 | The efficacy of bivalent and tetravalent HPV vaccination against cervical intraepithelial neoplasia and persistent HPV 16 and 18 infection | Abstract of presentation only |
| Schiller, 2012 | A review of clinical trials of human papillomavirus prophylactic vaccines | Not a systematic review |
| Araujo, 2013 | [Efficacy of Commercially Available Vaccines Against HPV Infection in Women: A Systematic Review and Meta-Analysis] | Duplicate |
| Miltz, 2013 | Systematic review and meta-analysis of L1-VLP-based human papillomavirus vaccine efficacy against anogenital pre-cancer in women with evidence of prior HPV exposure | Abstract of presentation only |
| Tomljenovic, 2013 | Human papillomavirus (HPV) vaccines as an option for preventing cervical malignancies: (how) effective and safe? | Not a systematic review |
| Yvonne, 2013 | Duration of protection after vaccination against human papillomavirus | Not a systematic review |
| Angelo, 2014 | Pooled analysis of large and long-term safety data from the human papillomavirus-16/18-AS04-adjuvanted vaccine clinical trial programme | Not a systematic review |
| Deleré, 2014 | The efficacy and duration of vaccine protection against human papillomavirus | Duplicate |
| DeVincenzo, 2014 | Long-term efficacy and safety of human papillomavirus vaccination | Not a systematic review |
| Erickson, 2014 | Update on vaccination clinical trials for HPV-related disease | Not a systematic review |
| Huang, 2014 | Can HPV vaccine have other health benefits more than cancer prevention? A systematic review of association between cervical HPV infection and preterm birth | Wrong intervention; wrong outcomes |
| BoTerning, 2015 | HPV vaccination status and changes in sexual behavior: a systematic review | Not a systematic review |
| Konstantyner, 2015 | Safety of human papillomavirus 6, 11, 16 and 18 (recombinant): systematic review and meta-analysis | Duplicate |
| Mariani, 2015 | Early direct and indirect impact of quadrivalent HPV (4HPV) vaccine on genital warts: a systematic review | Wrong study design |
| Stillo, 2015 | Safety of human papillomavirus vaccines: a review | Not a systematic review |
| Vichnin, 2015 | An overview of quadrivalent human papillomavirus vaccine safety: 2006 to 2015 | Not a systematic review |
| Alexandra, 2016 | Sociodemographic differences in human papillomavirus vaccine impact: a systematic review | Wrong study design |
| Brisson, 2016 | Population-level impact, herd immunity, and elimination after human papillomavirus vaccination: a systematic review and meta-analysis of predictions from transmission-dynamic models | Wrong outcomes |
| Hatice, 2016 | The efficacy of the human papillomavirus vaccine against cervical dysplasia and safety of the vaccine: a systematic review and meta-analysis | Not a systematic review |
| Jordão, 2016 | Adverse events following HPV vaccination: a systematic review | Not a systematic review |
| Mofrad, 2016 | The role of human papilloma virus (HPV) vaccines in prevention of cervical cancer | Not a systematic review |
| Thomas, 2016 | Efficacy, effectiveness and safety of vaccination against human papillomavirus in males | Not a systematic review |
| Ventimiglia, 2016 | Human papillomavirus infection and vaccination in males | Wrong outcomes |
| Yang, 2016 | Update on the new 9-valent vaccine for human papillomavirus prevention | Not a systematic review |
| Bissett, 2017 | Seropositivity to non-vaccine incorporated genotypes induced by the bivalent and quadrivalent HPV vaccines: a systematic review and meta-analysis | Wrong outcomes |
| Caroline, 2017 | Safety, efficacy and immunogenicity of therapeutic vaccines in the treatment of patients with high-grade cervical intraepithelial neoplasia (CIN 2/3) associated with human papillomavirus (HPV): a systematic review | Not a systematic review |
| Costa, 2017 | Safety of human papillomavirus 9-valent vaccine: a meta-analysis of randomized trials | Wrong comparator |
| D’Addario, 2017 | Two-dose schedules for human papillomavirus vaccine: systematic review and meta-analysis | Wrong comparator |
| Di Mario, 2017 | Corrigendum to “Are the two human papillomavirus vaccines really similar? A systematic review of available evidence: efficacy of the two vaccines against HPV” | Erratum |
| DiMario, 2017 | Corrigendum to “Are the two human papillomavirus vaccines really similar? A systematic review of available evidence: efficacy of the two vaccines against HPV” | Duplicate and erratum |
| Herney, 2017 | The effects of vaccination on papillomavirus infection prevalence and incidence: a systematic review and meta-analysis | Not a systematic review |
| Lars, 2017 | Benefits and harms of human papillomavirus vaccines: systematic review of industry clinical study reports and non-industry published and unpublished reports | Not a systematic review |
| Martinez-Lavin, 2017 | Erratum to: serious adverse events after HPV vaccination: a critical review of randomized trials and post-marketing case series | Erratum |
| Prudence, 2017 | Human papillomavirus prevalence in women following HPV vaccine introduction: a systematic review | Not a systematic review |
| Tine, 2017 | Therapeutic HPV vaccination and recurrent respiratory papillomatosis: a systematic review and meta-analysis | Not a systematic review |
| Anita, 2018 | Trends in genital warts due to the quadrivalent human papillomavirus vaccination: a meta-analysis | Not a systematic review |
| Arbyn, 2018 | Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors | Duplicate |
| Hutcherson, 2018 | Systematic review of clinical and pharmacoeconomic outcomes of the human papillomavirus nonavalent recombinant vaccine | Abstract of presentation only |
| Muusha, 2018 | Human papillomavirus prevalence among women following HPV vaccine introduction: a systematic review | Abstract of presentation only |
| Phillips, 2018 | Safety of human papillomavirus vaccines: an updated review | Not a systematic review |
| Schneider, 2018 | Therapeutic human papillomavirus vaccines in head and neck cancer: a systematic review of current clinical trials | Wrong intervention |
| Stacey, 2018 | A systematic review of human papillomavirus (HPV) vaccine in the treatment of HPV related vulval and vaginal intraepithelial neoplasia | Not a systematic review |
| Steben, 2018 | A review of the impact and effectiveness of the quadrivalent human papillomavirus vaccine: 10 years of clinical experience in Canada | Wrong study design |
| Yakely, 2018 | Impact and effectiveness of the human papillomavirus (HPV) vaccine on anogenital warts in United States and Canada: a systematic review | Duplicate |
| STUDY | REVIEW AIM OR PICO QUESTION | SEARCH STRATEGY | TIME INCLUDED IN LITERATURE SEARCH | INCLUSION CRITERIA | NO. OF STUDIES INCLUDED | TOTAL NO. OF PARTICIPANTS | PARTICIPANT DEMOGRAPHICS | HPV† VACCINE | COMPARATOR | REPORTED OUTCOMES | DATA ANALYSIS TYPE | POTENTIAL SOURCES OF CONFLICT OF INTEREST | AMSTAR 2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| La Torre and Colleagues, 200738x38La Torre, G., de Waure, C., Chiaradia, G., Mannocci, A., and Ricciardi, W. HPV vaccine efficacy in preventing persistent cervical HPV infection: a systematic review and meta-analysis. Vaccine. 2007; 25: 8352–8358 Google ScholarSee all References | Review scientific literature regarding HPV vaccine efficacy in preventing cervical persistent infection | PubMed, Embase, Cochrane Library; gave search terms; no language restriction | 1990-August 15, 2007 | RCTs‡; females; evaluated cervical persistent infection | 5 | 22,630 | Females aged 13-25 y | 1vHPV, 2vHPV, 4vHPV# | Placebo; HPV 11 vaccine, hepatitis A vaccine | Persistent (6 mo) cervical infection with HPV 16 | Meta-analysis | Did not report | Moderate |
| Rambout and Colleagues, 200749x49Rambout, L., Hopkins, L., Hutton, B., and Fergusson, D. Prophylactic vaccination against human papillomavirus infection and disease in women: a systematic review of randomized controlled trials. CMAJ. 2007; 177: 469–479 Google ScholarSee all References | Determine whether females who receive prophylactic HPV vaccination have a lower incidence of HPV persistent infection and precancerous lesions than females who are not vaccinated | MEDLINE, Embase, Cochrane Registry, Cochrane Library, Google Scholar, Clinical Trial Registry, Public Health Announcements, conference proceedings, manufacturer’s information; searched reference lists, bibliographies; no language restriction | 1950-2007 | RCTs; females; any HPV vaccine; any dosing; placebo or no vaccine comparator; only outcomes related to oncogenic strains | 6 | 40,323 | Females 15-25 y | 1vHPV, 2vHPV, 4vHPV | Placebo, unvaccinated | Cervical lesions; persistent HPV infections; external genital disease; adverse events | Meta-analysis | Yes | High |
| Agorastos and Colleagues, 200928x28Agorastos, T., Chatzigeorgiou, K., Brotherton, J.M., and Garland, S.M. Safety of human papillomavirus (HPV) vaccines: a review of the international experience so far. Vaccine. 2009; 27: 7270–7281 Google ScholarSee all References | Report the international experience on safety of prophylactic HPV vaccines to date | MEDLINE, bibliography of selected articles, conference abstracts, position statements, gray literature | Through January 31, 2009, for articles; through April 10, 2009 for other materials | Not stated | 13 published reports; 7 reports from public health agencies | > 60,000 | Females 12 y or older, multisite, multicountry | 2vHPV, 4vHPV | Placebo for prelicensure data; not stated for other studies | Local and systemic adverse events | Qualitative synthesis | Yes | Critically low |
| Medeiros and Colleagues, 200946x46Medeiros, L.R., Rosa, D.D., da Rosa, M.I., Bozzetti, M.C., and Zanini, R.R. Efficacy of human papillomavirus vaccines: a systematic quantitative review. Int J Gynecol Cancer. 2009; 19: 1166–1176 Google ScholarSee all References | Systematically review RCTs in which HPV vaccine was compared with placebo regarding safety, efficacy, and immunogenicity | MEDLINE, CANCERLIT, LILACS, Embase, Cochrane Library (2007 issue 2); included search terms; no language restrictions; searched reference lists | 1/1997-9/2007 | No language criteria; humans; placebo-controlled RCTs; females; excluded cohort and case-control studies and studies that did not describe final histologic results | 6 | 47,236 | Females aged 9-26 y | 2vHPV, 4vHPV | Placebo, hepatitis A vaccine | Adverse events; condyloma; VIN†† I-III; VaIN‡‡ I-III; CIN 1-3 | Meta-analysis | No | Moderate |
| Yancey and Colleagues, 201057x57Yancey, A.M., Pitlick, J.M., and Forinash, A.B. The prophylactic role for the human papillomavirus quadrivalent vaccine in males. Annal Pharmacother. 2010; 44: 1314–1318 Google ScholarSee all References | Evaluate immunogenicity, efficacy, and safety of the 4vHPV vaccine in males | PubMed and searched reference lists; English language only | 1966-March 2010 | HPV 4vHPV vaccine; males | 3 | 1,147 | Males aged 9-17 y, multicenter | 4vHPV | Placebo | Immunogenicity, external genital lesions, warts, genital intraepithelial neoplasia, adverse events | Qualitative synthesis | No | Critically low |
| Lu and Colleagues, 201139x39Lu, B., Kumar, A., Castellsagué, X., and Giuliano, A.R. Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review & meta-analysis. BMC Infect Dis. 2011; 11 () Google ScholarSee all References | Provide a comprehensive assessment of vaccine safety and efficacy against multiple virologic and clinical end points | MEDLINE, Cochrane Library, Cochrane Central Register of Controlled Trials; included search terms; searched bibliographies and hand searched conference abstract books; English only | 2006-August 31, 2009 | RCTs; English language; on L1 VLP-based HPV vaccines; female participants | 13 studies representing 7 RCTs | 44,142 | Females aged 15-45 y, multicenter | 1vHPV, 2vHPV, 4vHPV | Placebo, hepatitis A vaccine, hepatitis B vaccine | Persistent infection (6 mo), CIN 1+, adverse events | Meta-analysis | Yes | Low |
| Malagón and Colleagues, 201243x43Malagón, T., Drolet, M., Boily, M.C. et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012; 12: 781–789 Google ScholarSee all References | Summarize and compare evidence from clinical trials about the cross-protective efficacy of the 2vHPV and 4vHPV vaccines in HPV-naïve populations | MEDLINE, Embase; included search terms; searched reference lists; searched abstracts of HPV conferences; vaccine Web sites; contacted manufacturers for unpublished results; no mention of language restriction | Through January 2012 | RCTs; 2vHPV or 4vHPV vaccine; any population; reported on efficacy against cervical or genital infection or disease | 12 (4 RCTs) | 22,559 | Females aged 15-26 y, multicenter | 2vHPV, 4vHPV | Placebo, hepatitis A vaccine | Persistent infection (6 mo), CIN 2+ | Meta-analysis | Yes | Moderate |
| Rey-Ares and Colleagues, 201250x50Rey-Ares, L., Ciapponi, A., and Pichon-Riviere, A. Efficacy and safety of human papilloma virus vaccine in cervical cancer prevention: systematic review and meta-analysis. Arch Argent Pediatr. 2012; 110: 483–489 Google ScholarSee all References | Conduct a systematic review and meta-analysis to evaluate the efficacy and safety of HPV vaccines in preventing CIN grades 2 and 3, AIS## (CIN 2+) and cervical cancer | MEDLINE, Cochrane Central Registry of Controlled Trials, Database of Abstract Reviews of Effects, National Health Service Economic Evaluation Database, LILACS, Embase, “generic Internet search”; no language restriction | Through July 2011 | Clinical trials; 2vHPV or tetravalent vaccines; included CIN 2+ lesions or cervical cancer as outcomes | 4 articles on 3 trials | 190,534 | Healthy females aged 15-26 y | 2vHPV, 4vHPV | Placebo, hepatitis A vaccine | CIN 2+, AIS, cervical cancer, adverse events | Meta-analysis | Did not report | Low |
| Araujo and Colleagues, 201329x29Araujo, S.C., Caetano, R., Braga, J.U., and Costa e Silva, F.V. Efficacy of commercially available vaccines against HPV infection in women: a systematic review and meta-analysis [in Portuguese]. Cad Saude Publica. 2013; 29: S32–S44 Google ScholarSee all References | Determine the efficacy of 2vHPV and quadrivalent vaccine to reduce the risk of CIN 2 and CIN 3 in females | MEDLINE, LILACS, Cochrane Library; Professional librarian’s help with search terms; no language restriction | 2000-November 2009 | RCTs; efficacy of commercially available 2vHPV or 4vHPV HPV vaccines; females only | 6 | 41,750 | Females aged 12-45 y | 2vHPV, 4vHPV | Placebo, hepatitis A vaccine | Persistent infection, CIN 2+, AIS | Meta-analysis | No | Moderate |
| Macartney and Colleagues, 201341x41Macartney, K.K., Chiu, C., Georgousakis, M., and Brotherton, J.M.L. Safety of human papillomavirus vaccines: a review. Drug Saf. 2013; 36: 393–412 Google ScholarSee all References | Assess all available published safety data on both HPV vaccines | MEDLINE, Embase; no language restriction; searched reference lists bibliographies; searched Internet | Through May 2012 | Reported safety data from 2 available HPV vaccines | 12 trials; 21 case series; 4 post licensure studies; 8 post licensure population-based studies | > 21,000 | Both sexes, aged 9-26 y | 2vHPV, 4vHPV | Unvaccinated | Adverse events | Qualitative synthesis | No | Critically low |
| Couto and Colleagues, 201432x32Couto, E., Sæterdal, I., Juvet, L.K., and Klemp, M. HPV catch-up vaccination of young women: a systematic review and meta-analysis. BMC Public Health. 2014; 14 () Google ScholarSee all References | Determine efficacy of vaccinating females against HPV starting at 16 y or older | Ovid MEDLINE, Embase, Cochrane Central Register of Controlled Trials, PubMed, ISI Web of Science, Google Scholar; search strategy in appendix; contacted pharmaceutical companies for additional information; no mention of language restriction | 1999-October 2012 | RCTs; examined efficacy of HPV vaccination in females 16 y or older | 46 articles on 13 studies | 40,800 | Healthy females with 0-6 sexual partners, aged 16-45 y | 1vHPV, 2vHPV, 4vHPV | Placebo, hepatitis A vaccine | Mortality; CIN 2+; genital warts; VIN 2+; VaIN 2+; condyloma; serious adverse events | Meta-analysis | No | Low |
| Deleré and Colleagues, 201433x33Deleré, Y., Wichmann, O., Klug, S.J. et al. The efficacy and duration of vaccine protection against human papillomavirus. Dtsch Arztebl Int. 2014; 111: 584–591 () Google ScholarSee all References | Evaluate the duration of protection after HPV vaccination and clarify whether people vaccinated in childhood are protected against HPV years later | MEDLINE, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Database of Abstract Reviews of Effects; full search strategy in eAppendix; no restriction based on publication status or language | Through November 19, 2013 | Immunization with licensed vaccine according to schedule 0-1-(-2)-6 mo or similar, without booster doses after primary vaccination; must have comparison group | 15 | 46,436 | Females aged 9-26 y, generally healthy, any country | 2vHPV, 4vHPV | Placebo, hepatitis A vaccine | Incident or persistent HPV infection; CIN 2+ | Meta-analysis | Yes | Moderate |
| Goncalves and Colleagues, 201436x36Goncalves, A.K., Cobucci, R.N., Rodrigues, H.M., de Melo, A.G., and Giraldo, P.C. Safety, tolerability and side effects of human papillomavirus vaccines: a systematic quantitative review. Braz J Infect Dis. 2014; 18: 651–659 Google ScholarSee all References | Evaluate safety and adverse events of HPV vaccines. | PubMed, Embase, Scientific Electronic Library Online, CANCERLIT; gave heading terms and text words; no language restriction | Through March 2013 | Double-blind RCT; evaluate 2vHPV or 4vHPV HPV vaccines; participants aged > 9 y; excluded pregnant females and those at high risk of contracting HPV | 12 | 29,540 | Predominantly but not exclusively females; aged 9-45 y | 2vHPV, 4vHPV | Placebo, hepatitis A vaccine | Adverse events | Meta-analysis | No | Low |
| Miltz and Colleagues, 201447x47Miltz, A., Price, H., Shahmanesh, M., Copas, A., and Gilson, R. Systematic review and meta-analysis of L1-VLP-based human papillomavirus vaccine efficacy against anogenital pre-cancer in women with evidence of prior HPV exposure. PLoS One. 2014; 9 Google ScholarSee all References | Review the efficacy of the HPV vaccine in females with evidence of prior HPV exposure | MEDLINE; Embase; Web of Science; PubMed; Cochrane Central Register of Controlled Trials; English only | Through August 30, 2013 | RCT and post-RCT; investigated HPV vaccine efficacy against associated CIN 3+ or VIN 2-3/VaIN 2-3; studies where females had prior exposure to vaccine-type HPV exposure | 5 | 8,387 | Females mean aged 20-43 y | 1vHPV, 2vHPV, 4vHPV | Placebo, hepatitis A vaccine | CIN 3+, VIN 2+ | Meta-analysis | No | Moderate |
| Coelho and Colleagues, 201531x31Coelho, P.L., da Silva Calestini, G.L., Alvo, F.S., de Moura Freitas, J.M., Castro, P.M., and Konstantyner, T. Safety of human papillomavirus 6, 11, 16 and 18 (recombinant): wystematic review and meta-analysis [in Portuguese]. Rev Paul Pediatr. 2015; 33: 474–482 Google ScholarSee all References | Identify and quantify the adverse effects associated with the recombinant HPV (types 6, 11, 16 and 18) vaccine in adolescents | PubMed, LILACS, Scientific Electronic Library Online; searched for HPV vaccines and adverse effects; no language or date restrictions | Through April 2014 | RCTs; adolescents; no patients with cervical diseases, HIV, or had already received HPV vaccine | 14 | 40,458 | Females aged 9-45 y; males aged 9-26 y | 2vHPV, 4vHPV | Placebo, non-HPV vaccine, different HPV vaccine dose schedule or type | Adverse events | Meta-analysis | No | Low |
| Di Mario and Colleagues, 201534x34Di Mario, S., Basevi, V., Lopalco, P.L., Balduzzi, S., D’Amico, R., and Magrini, N. Are the two human papillomavirus vaccines really similar? A systematic review of available evidence: efficacy of the two vaccines against HPV. J Immunol Res. 2015; 2015: 13 Google ScholarSee all References | Assess the efficacy of the 2vHPV and 4vHPV vaccines against cervical cancer | Cochrane Library, MEDLINE, Embase; also searched Internet for prepublication presentations; experts and vaccine manufacturers contacted; searched reference lists; no language or time restriction | Through March 2014 | RCTs; compared HPV 2vHPV or 4vHPV vaccines with placebo or any other control; must include females | 9 articles based on 5 trials | 38,419 | Healthy, nonpregnant females aged 15-26 y, multiple sites | 2vHPV, 4vHPV | Placebo, hepatitis A vaccine | CIN 2+, CIN 3+, AIS | Meta-analysis | No | High |
| Luo and Colleagues, 201540x40Luo, W., Zhang, S.H., Zhou, Y.Z. et al. Safety and immunogenicity of quadrivalent HPV vaccine: a meta-analysis. Chin J Evid Based Med. 2015; 15: 47–53 Google ScholarSee all References | Systematically review the safety and immunogenicity of 4vHPV vaccine among healthy population | PubMed, Embase, Chinese Biomedical Literature Database, Cochrane Library, China National Knowledge Infrastructure, Web of Science, Wang Fang Data; searched reference lists; included search terms; no language restriction | Through October 2013 | RCTs | 9 | 39,688 | Both sexes, aged 9-45 y | 4vHPV | Placebo | Adverse events, immunogenicity | Meta-analysis | Did not report | Moderate |
| Sangar and Colleagues, 201551x51Sangar, V.C., Ghongane, B.B., Mathur, G., and Chowdhary, A.S. Safety and adverse events of prophylactic HPV vaccines among healthy women: a systematic review & meta analysis. Int J Pharm Sci Res. 2015; 6: 1779–1791 Google ScholarSee all References | Evaluate HPV vaccine safety | MEDLINE, Cochrane Library, Cochrane Central Register of Controlled Trials; included search terms; no mention of searching reference lists or gray literature; English only | June 1996-November 2014 | Double-blind RCTs; healthy females; 2vHPV or 4vHPV vaccines | 4 | 1,427 | Females aged 10-35 y; Asian and African sites | 2vHPV | Placebo | Adverse events | Meta-analysis | Yes | Critically low |
| Tan and Colleagues, 201554x54Tan, P., Wang, X., Wei, S. et al. Efficacy and safety of prophylactic human papillomavirus vaccination in healthy males: a meta-analysis. Rev Med Microbiol. 2015; 26: 143–153 Google ScholarSee all References | Systematically assess the current evidence regarding the efficacy and safety of HPV vaccination in healthy males | PubMed, Embase, MEDLINE, Web of Science, ClinicalTrials.gov; restricted to English; searched reference lists; looked for trials and conference abstracts | Through November 2014 | Males; healthy study population; clinical trials, prospective cohort studies or vaccine intervention studies; ≥ 10 patients/study | 3 safety, 8 in total | 4,139 for safety, 6,079 in total | Males aged 9-55 y | 1vHPV, 2vHPV, 4vHPV | Placebo, hepatitis B vaccine | Immunogenicity, adverse events | Meta-analysis | No | Critically low |
| Garland and Colleagues, 201635x35Garland, S.M., Kjaer, S.K., Muñoz, N. et al. Impact and effectiveness of the quadrivalent human papillomavirus vaccine: a systematic review of 10 years of real-world experience. Clin Infect Dis. 2016; 63: 519–527 Google ScholarSee all References | Quantify reported effectiveness and impact of 4vHPV vaccination on HPV infection, anogenital warts, and cervical cytologic and histologic abnormalities | PubMed and Embase; full search terms in eAppendix; no language restriction; searched reference lists | January 1, 2007-February 29, 2016 | RCT or observational studies; must relate to 2vHPV or 4vHPV HPV vaccines | 58 | Millions | Primarily but not exclusively females; 9 y or older | 2vHPV, 4vHPV | Prevaccination period, unvaccinated | HPV June 11, 2018 infection prevalence; genital warts; cervical cytologic and histologic abnormalities | Qualitative synthesis | Yes | Critically low |
| Macki and Colleagues, 201642x42Macki, M. and Dabaja, A.A. Literature review of vaccine-related adverse events reported from HPV vaccination in randomized controlled trials. Basic Clin Androl. 2016; 26: 16 Google ScholarSee all References | Determine safety of the HPV vaccine. | PubMed; search term was human papillomavirus vaccine, no language restriction mentioned; no searched reference lists mentioned | Through October 2014 | RCTs; compared 2vHPV or 4vHPV vaccines with control (trials with hepatitis A or B comparators were excluded) | 13 | 31,289 | All sexes but predominantly females; multicenter; aged 9-45 y | 2vHPV, 4vHPV | Placebo | Adverse events | Qualitative synthesis | No | Critically low |
| Martínez-Lavín and Colleagues, 201745x45Martínez-Lavín, M. and Amezcua-Guerra, L. Serious adverse events after HPV vaccination: a critical review of randomized trials and post-marketing case series. Clin Rheumatol. 2017; 36: 2169–2178 Google ScholarSee all References | Critically review HPV vaccine serious adverse events described in prelicensure RTs and post marketing case series | PubMed; search term was HPV vaccine; no mention of language; no search of reference lists | Through January 31, 2017 | RCTs or post marketing studies | 16 RCTs, 12 post marketing case series | 60,729 | All sexes, aged 9-45 y, multicenter | 2vHPV, 4vHPV, 9vHPV | Placebo, hepatitis A vaccine | Adverse events | Qualitative synthesis | No | Critically low |
| Ogawa and Colleagues, 201748x48Ogawa, Y., Takei, H., Ogawa, R., and Mihara, K. Safety of human papillomavirus vaccines in healthy young women: a meta-analysis of 24 controlled studies. J Pharm Health Care Sci. 2017; 3 Google ScholarSee all References | Evaluate the safety of the HPV vaccine in healthy young females | MEDLINE, Cochrane Central Register of Controlled Trials, Japana Centra Revuo Medicina, Pharmaceuticals and Medicines Devices Agency; no language restriction | 1966-February 2017 | Prospective controlled studies; young, healthy females; 2vHPV, 4vHPV, or 9vHPV | 22 articles, 24 studies | 23,570 | Healthy females aged 12-37 y | 2vHPV, 4vHPV, 9vHPV | Placebo, hepatitis A vaccine, hepatitis B vaccine, other HPV vaccine (2vHPV compared with 4vHPV, 4vHPV compared with 9vHPV) | Adverse events | Meta-analysis | No | Low |
| Setiawan and Colleagues, 201752x52Setiawan, D., Luttjeboer, J., Pouwels, K.B., Wilschut, J.C., and Postma, M.J. Immunogenicity and safety of human papillomavirus (HPV) vaccination in Asian populations from six countries: a meta-analysis. Jpn J Clin Oncol. 2017; 47: 265–276 Google ScholarSee all References | Investigate the immunogenicity and safety profiles of HPV vaccines among both uninfected and infected populations in Asian countries | PubMed, Embase, Cochrane Library, ClinicalTrials.gov; gave search terms; no mention of searching reference lists or gray literature; English only | Through November 21, 2014 | RCTs; conducted in Asia; immunogenicity or safety as outcomes | 10 | 9,400 | Both sexes but predominantly female; aged 9-45 y; multiple Asian countries | 2vHPV, 4vHPV | Placebo, hepatitis A vaccine | Immunogenicity and adverse events | Meta-analysis | No | Critically low |
| Signorelli and Colleagues, 201753x53Signorelli, C., Odone, A., Ciorba, V. et al. Human papillomavirus 9-valent vaccine for cancer prevention: a systematic review of the available evidence. Epidemiol Infect. 2017; 145: 1962–1982 Google ScholarSee all References | Systematically assess all available evidence from RCTs on 9vHPV | MEDLINE, Embase, Cochrane Library registered clinical trials; searched reference lists; consulted with experts; search strategies in supplement; English language only | Through August 25, 2016 | Clinical trials; 9vHPV vaccine; any age group, study population, comparison, dose, or outcome | 10 included, 1 looked at vaccine versus placebo | 28,608 in total, 924 from single trial with placebo control | Males and females aged 9-26 y | 9vHPV | Placebo | Immunogenicity; high-grade cervical, vulvar, and vaginal disease or cancer; adverse events | Qualitative synthesis | No | Critically low |
| Tejada and Colleagues, 201755x55Tejada, R.A., Vargas, K.G., Benites-Zapata, V., Mezones-Holguín, E., Bolaños-Díaz, R., and Hernandez, A.V. Human papillomavirus vaccine efficacy in the prevention of anogenital warts: systematic review and meta-analysis. Salud Publica Mex. 2017; 59: 84–94 Google ScholarSee all References | Summarize available evidence on the efficacy of HPV vaccines in preventing nononcological lesions | MEDLINE, Embase, Cochrane Library, Scopus, LILACS, SciELO, Web of Knowledge; reviewed conference abstracts, National Institutes of Health and Europe clinical registries, searched reference lists; search strategies available online; no language restriction | Through July 2015 | RCTs; nononcological lesions evaluated as outcome | 6 | 27,079 | Both sexes, adults, multicenter | 2vHPV, 4vHPV | Placebo | Anogenital warts | Meta-analysis | No | High |
| Arbyn and Colleagues, 201830x30Arbyn, M., Xu, L., Simoens, C., and Martin-Hirsch, P.P.L. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018; 2018 Google ScholarSee all References | Evaluate the harms and protection of HPV vaccines against cervical precancer and HPV 16/18 infection in adolescent and older females | The Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE Search terms in appendixes No language restriction | 2002-July 2017 | Phase II and III RCTs; female participants; placebo comparator | 26 | 73,428 | Females aged 15-45 y | 1vHPV, 2vHPV, 4vHPV | Placebo with no active product or non–HPV vaccine | CIN 2+; AIS; adverse events; HPV infection (16 and 18 or 6, 11, 16, and 18) | Meta-analysis | Did not report | Moderate |
| Harder and Colleagues, 201837x37Harder, T., Wichmann, O., Klug, S.J., van der Sande, M.A.B., and Wiese-Posselt, M. Efficacy, effectiveness and safety of vaccination against human papillomavirus in males: a systematic review. BMC Med. 2018; 16: 110–127 Google ScholarSee all References | Assess the currently available evidence on the efficacy, effectiveness, and safety of HPV vaccination in males | MEDLINE, Embase, Cochrane Central Register of Controlled Trials, ClinicalTrials.gov, conference abstracts, searched reference lists; no language or publication status restriction | Through April 18, 2017 | Males; report clinically relevant outcome | 7 | 5,294 | Men aged 12-76 y, multicenter, multicountry | 4vHPV | Placebo, unvaccinated | Incident or persistent oral or anogenital infection; anogenital warts; anal intraepithelial neoplasia 2+, penile intraepithelial neoplasia 2+, severe adverse events | Qualitative synthesis | No | Moderate |
| Markowitz and Colleagues, 201844x44Markowitz, L.E., Drolet, M., Perez, N., Jit, M., and Brisson, M. Human papillomavirus vaccine effectiveness by number of doses: systematic review of data from national immunization programs. Vaccine. 2018; 36: 4806–4815 Google ScholarSee all References | Systematically review HPV vaccine effectiveness by number of doses. | MEDLINE; Embase; provided search terms; no language restriction; no mention of searched reference lists | January 1, 2007-June 15, 2017 | Reported effectiveness of HPV vaccination on infection, warts, or cervical abnormalities; assessed effectiveness of HPV vaccination by number of doses; excluded RCTs | 14 | 3,104,053 | Aged 9-26 y, multicountry | 2vHPV, 4vHPV | Other number of doses of HPV vaccine | Vaccine-type HPV infection; anogenital warts; CIN 1-3+; AIS | Qualitative synthesis | No | Low |
| Yakely and Colleagues, 201856x56Yakely, A.E., Avni-Singer, L., Oliveira, C.R., and Niccolai, L.M. Human papillomavirus vaccination and anogenital warts: a systematic review of impact and effectiveness in the United States. Sex Transm Dis. 2018; 46: 213–220 Google ScholarSee all References | Assess real-world impact and effectiveness of the HPV vaccine as it relates to anogenital warts | PubMed, MEDLINE, Embase; search terms in Supplement; English language restriction; no reference list search | January 1, 2006-March 12, 2018 | Must include 4vHPV or 9vHPV vaccine; must include anogenital warts as outcome; US setting | 3 effectiveness, 8 in total | 109,123,079 for impact studies; 452,736 for effectiveness studies | Both sexes, 9 y or older, entirely US-based | 4vHPV | Unvaccinated | Anogenital warts | Qualitative synthesis | Yes | Critically low |
| STUDY | SEROCONVERSION | HPV INCIDENT INFECTION EFFICACY | HPV PERSISTENT (> 6 MO) INFECTION EFFICACY | HPV ORAL INFECTION EFFICACY | HPV-RELATED WART EFFICACY | HPV-RELATED PRECANCEROUS LESIONS | HPV-RELATED ORAL CANCER | AE† RATE |
|---|---|---|---|---|---|---|---|---|
| La Torre and Colleagues, 200738x38La Torre, G., de Waure, C., Chiaradia, G., Mannocci, A., and Ricciardi, W. HPV vaccine efficacy in preventing persistent cervical HPV infection: a systematic review and meta-analysis. Vaccine. 2007; 25: 8352–8358 Google ScholarSee all References | Not evaluated | Not evaluated | HPV 16 persistent cervical infection: RR,‡ 0.10 (95% CI, 0.07 to 0.15); HPV 18 persistent cervical infection: RR, 0.22 (95% CI, 0.13 to 0.38) | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated |
| Rambout and Colleagues, 200749x49Rambout, L., Hopkins, L., Hutton, B., and Fergusson, D. Prophylactic vaccination against human papillomavirus infection and disease in women: a systematic review of randomized controlled trials. CMAJ. 2007; 177: 469–479 Google ScholarSee all References | Not evaluated | Not evaluated | Peto-OR, 0.14 (95% CI, 0.10 to 0.19) per protocol; Peto-OR,0.22 (95% CI, 0.18 to 0.27) per ITT# | Not evaluated | Peto-OR, 0.13 (95% CI, 0.08–0.22) per protocol; Peto-OR, 0.30 (95% CI, 0.22 to 0.43) per ITT | Per protocol: CIN 2+: Peto-OR, 0.14 (95% CI, 0.09 to 0.21) Any CIN: Peto-OR, 0.13 (95% CI, 0.09 to 0.20) Modified ITT: CIN 2+: Peto-OR, 0.52 (95% CI, 0.43 to 0.63) Any CIN: Peto-OR, 0.36 (95% CI, 0.29 to 0.45) | Not evaluated | More than 1 AE: Peto-OR, 1.00 (95% CI, 0.87 to 1.14) Death: Peto-OR, 0.91 (95% CI, 0.39 to 2.14) |
| Medeiros and Colleagues, 200946x46Medeiros, L.R., Rosa, D.D., da Rosa, M.I., Bozzetti, M.C., and Zanini, R.R. Efficacy of human papillomavirus vaccines: a systematic quantitative review. Int J Gynecol Cancer. 2009; 19: 1166–1176 Google ScholarSee all References | Not evaluated | HPV 16/18: OR, 0.09 (95% CI, 0.05 to 0.15) | HPV 16/18: OR, 0.26 (95% CI, 0.07 to 0.99) | Not evaluated | OR, 0.24 (95% CI, 0.17 to 0.33) | 2vHPV†† vaccine on low- or high-grade SIL‡‡: OR, 0.07 (95% CI, 0.04 to 0.14); 4vHPV vaccine on low-grade SIL: OR, 0.13 (95% CI, 0.01 to 2.04); 4vHPV vaccine on high-grade SIL: OR, 0.54 (95% CI, 0.30 to 0.98); adenocarcinoma in situ: OR, 0.45 (95% CI, 0.12 to 1.73); VIN 1 or VaIN## 1: OR, 0.37 (95% CI, 0.15 to 0.96); VIN 2-3 or VaIN 2-3: OR, 0.38 (95% CI, 0.14 to 1.08) | Not evaluated | 2vHPV: injection site: OR, 1.74 (95% CI, 1.27 to 2.40) Systemic: OR, 1.18 (95% CI, 0.07 to 1.99) Serious AE: OR, 1.05 (95% CI, 0.91 to 1.21) Death: not estimable Total: OR, 1.35 (95% CI, 1.05 to 1.73) 4vHPV: any AE: OR, 1.16 (95% CI, 0.94 to 1.43) |
| Lu and Colleagues, 201139x39Lu, B., Kumar, A., Castellsagué, X., and Giuliano, A.R. Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review & meta-analysis. BMC Infect Dis. 2011; 11 () Google ScholarSee all References | Not evaluated | Not evaluated | HPV 16: ITT RR, 0.15 (95% CI, 0.10 to 0.23) and per-protocol RR, 0.06 (95% CI, 0.04 to 0.09); HPV 18: ITT RR, 0.24 (95% CI, 0.14 to 0.42) and per-protocol RR, 0.05 (95% CI, 0.03 to 0.09) | Not evaluated | Not evaluated | ITT CIN 2+ associated with HPV 16: RR, 0.47 (95% CI, 0.36 to 0.61) or 53% efficacy; perprotocol CIN 2+ associated with HPV 16 RR, 0.04 (95% CI, 0.01 to 0.11) or 96% efficacy; ITT CIN 2+ associated with HPV 18: RR, 0.16 (95% CI, 0.08 to 0.34) or 84% efficacy; per protocol CIN 2+ associated with HPV 18: RR, 0.10 (95% CI, 0.03 to 0.38) or 90% efficacy | Not evaluated | Serious AE: RR, 1.00 (95% CI, 0.91 to 1.09); injection-related serious AE: RR, 1.82 (95% CI, 0.79 to 4.20) |
| Rey-Ares and Colleagues, 201250x50Rey-Ares, L., Ciapponi, A., and Pichon-Riviere, A. Efficacy and safety of human papilloma virus vaccine in cervical cancer prevention: systematic review and meta-analysis. Arch Argent Pediatr. 2012; 110: 483–489 Google ScholarSee all References | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | CIN 2+ associated with HPV 16: RR, 0.45 (95% CI, 0.38 to 0.54); CIN 2+ associated with HPV 18: RR, 0.14 (95% CI, 0.08 to 0.25) | Not evaluated | Not evaluated |
| Araujo and Colleagues, 201329x29Araujo, S.C., Caetano, R., Braga, J.U., and Costa e Silva, F.V. Efficacy of commercially available vaccines against HPV infection in women: a systematic review and meta-analysis [in Portuguese]. Cad Saude Publica. 2013; 29: S32–S44 Google ScholarSee all References | Not evaluated | Not evaluated | RR, 0.07 (95% CI, 0.03 to 0.16) by protocol; RR, 0.52 (95% CI, 0.42 to 0.65) by ITT | Not evaluated | Not evaluated | CIN 2: RR, 0.03 (95% CI, 0.01 to 0.1) by protocol; RR, 0.37 (95% CI, 0.29 to 0.48) by ITT; CIN 3: RR, 0.04 (95% CI, 0.01 to 0.11) by protocol; RR, 0.58 (95% CI, 0.45 to 0.74) by ITT | Not evaluated | Not evaluated |
| Couto and Colleagues, 201432x32Couto, E., Sæterdal, I., Juvet, L.K., and Klemp, M. HPV catch-up vaccination of young women: a systematic review and meta-analysis. BMC Public Health. 2014; 14 () Google ScholarSee all References | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Any HPV type: RR, 0.38 (95% CI, 0.31 to 0.47), GRADE: high; HPV 6/11: RR, 0.28 (95% CI, 0.12 to 0.65), GRADE: high | CIN 2+ any HPV type at 4 y: RR, 0.8 (95% CI, 0.62 to 1.02), GRADE: moderate; VIN 2+ and VaIN 2+ any HPV type at 4 y: RR, 0.49 (95% CI, 0.32 to 0.76), GRADE: moderate; VIN 2+ and VaIN 2+ HPV-related at 4-5 y: RR, 0.72 (95% CI, 0.03 to 15.02), GRADE: low | Not evaluated | Serious AE at > 7 mo: RR, 0.99 (95% CI, 0.91 to 1.08), GRADE: moderate |
| Deleré and Colleagues, 201433x33Deleré, Y., Wichmann, O., Klug, S.J. et al. The efficacy and duration of vaccine protection against human papillomavirus. Dtsch Arztebl Int. 2014; 111: 584–591 () Google ScholarSee all References | Not evaluated | Incident HPV 16/18 infections RR, 0.17 (95% CI, 0.10 to 0.30), GRADE: high | HPV 16/18 RR, 0.10 (95% CI, 0.05 to 0.21), GRADE: high | Not evaluated | Not evaluated | CIN 2+ (any HPV type at median follow-up 27 mo) RR, 0.16 (95% CI, 0.05 to 0.50), GRADE: moderate; CIN 3+ (any HPV type at median follow-up 27 mo) RR, 0.06 (95% CI, 0.02 to 0.17), GRADE: high | Not evaluated | Not evaluated |
| Goncalves and Colleagues, 201436x36Goncalves, A.K., Cobucci, R.N., Rodrigues, H.M., de Melo, A.G., and Giraldo, P.C. Safety, tolerability and side effects of human papillomavirus vaccines: a systematic quantitative review. Braz J Infect Dis. 2014; 18: 651–659 Google ScholarSee all References | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | HPV 16/18: injection site pain OR, 3.29 (95% CI, 3.00 to 3.60); redness OR, 2.41 (95% CI, 2.17 to 2.68); swelling OR, 3.14 (95% CI, 2.79 to 3.53); fever OR, 1.21 (95% CI, 1.03 to 1.42); local symptoms OR, 2.33 (95% CI, 1.61 to 3.36); fatigue OR, 1.29 (95% CI, 1.18 to 1.42); gastrointestinal symptoms OR, 1.13 (95% CI, 1.00 to 1.28); headache OR, 1.17 (95% CI, 1.06 to 1.28); myalgia OR, 1.97 (95% CI, 1.77 to 2.20); arthralgia OR, 1.40 (95% CI, 1.20 to 1.64); general symptoms OR, 1.07 (95% CI, 0.82 to 1.41); HPV 6/11/16/18: pain OR, 2.88 (95% CI, 2.42 to 3.43); swelling OR, 2.65 (95% CI, 2.04 to 3.44); general symptoms OR, 1.11 (95% CI, 1.00 to 1.23) |
| Miltz and Colleagues, 201447x47Miltz, A., Price, H., Shahmanesh, M., Copas, A., and Gilson, R. Systematic review and meta-analysis of L1-VLP-based human papillomavirus vaccine efficacy against anogenital pre-cancer in women with evidence of prior HPV exposure. PLoS One. 2014; 9 Google ScholarSee all References | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | CIN 3+ associated with 16/18: OR, 0.90 (95% CI, 0.56 to 1.44); VIN 2-3/VaIN 2-3 associated with 16/18: OR, 2.25 (95% CI, 0.78 to 6.50) | Not evaluated | Not evaluated |
| Coelho and Colleagues, 201531x31Coelho, P.L., da Silva Calestini, G.L., Alvo, F.S., de Moura Freitas, J.M., Castro, P.M., and Konstantyner, T. Safety of human papillomavirus 6, 11, 16 and 18 (recombinant): wystematic review and meta-analysis [in Portuguese]. Rev Paul Pediatr. 2015; 33: 474–482 Google ScholarSee all References | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Local pain: RD,†† 0.11 (95% CI, 0.09 to 0.13); edema: RD, 0.08 (95% CI, 0.06 to 0.09); erythema: RD, 0.05 (95% CI, 0.04 to 0.07); fever: RD, 0.02 (95% CI, 0.01 to 0.03) |
| Di Mario and Colleagues, 201534x34Di Mario, S., Basevi, V., Lopalco, P.L., Balduzzi, S., D’Amico, R., and Magrini, N. Are the two human papillomavirus vaccines really similar? A systematic review of available evidence: efficacy of the two vaccines against HPV. J Immunol Res. 2015; 2015: 13 Google ScholarSee all References | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | CIN 2: hazard ratio, 0.74 (95% CI, 0.61 to 0.89); CIN 3: hazard ratio, 0.68 (95% CI, 0.44 to 1.06) | Not evaluated | Not evaluated |
| Luo and Colleagues, 201540x40Luo, W., Zhang, S.H., Zhou, Y.Z. et al. Safety and immunogenicity of quadrivalent HPV vaccine: a meta-analysis. Chin J Evid Based Med. 2015; 15: 47–53 Google ScholarSee all References | HPV 6 OR, 128.54 (95% CI, 37.22 to 443.9); HPV 11 OR, 89.6 (95% CI, 32.53 to 246.03); HPV 16 OR, 303.92 (95% CI, 46.41 to 1990.23); HPV 18 OR, 97.17 (95% CI, 33.91 to 278.40) | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Injection site: swelling and red spots: RR, 1.22 (95% CI, 1.13 to 1.32); systemic AE: RR, 1.03 (95% CI, 0.99 to 1.07); serious AE: RR, 1.06 (95% CI, 0.75 to 1.50) |
| Sangar and Colleagues, 201551x51Sangar, V.C., Ghongane, B.B., Mathur, G., and Chowdhary, A.S. Safety and adverse events of prophylactic HPV vaccines among healthy women: a systematic review & meta analysis. Int J Pharm Sci Res. 2015; 6: 1779–1791 Google ScholarSee all References | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Pain at injection site: RR, 1.17 (95% CI, 1.10 to 1.25); redness at injection site: RR, 2.30 (95% CI, 1.14 to 4.62); swelling at injection site: RR, 1.84 (95% CI, 1.47 to 2.3); arthralgia: RR, 1.39 (95% CI, 1.09 to 1.78); fatigue: RR, 1.18 (95% CI, 1.02 to 1.36); fever: RR, 1.07 (95% CI, 0.90 to 1.29); gastrointestinal: RR, 1.11 (95% CI, 0.89 to 1.4); headache: RR, 0.99 (95% CI, 0.85 to 1.14); myalgia: RR, 1.40 (95% CI, 1.16 to 1.68); rash: RR, 1.13 (95% CI, 0.73 to 1.73); urticaria: RR, 1.29 (95% CI, 0.89 to 1.88); new onset of chronic disease: RR, 0.56 (95% CI, 0.28 to 1.10); medically significant condition: RR, 0.93 (95% CI, 0.65 to 1.34); serious AE: RR, 0.66 (95% CI, 0.35 to 1.27) |
| Tan and Colleagues, 201554x54Tan, P., Wang, X., Wei, S. et al. Efficacy and safety of prophylactic human papillomavirus vaccination in healthy males: a meta-analysis. Rev Med Microbiol. 2015; 26: 143–153 Google ScholarSee all References | HPV 6 seroconversion rate: 99.5% (95% CI, 98.9% to 100%); HPV 11 seroconversion rate: 99.7% (95% CI, 99.4% to 100%); HPV 16 seroconversion rate: 99.6% (95% CI, 99.2% to 100%); HPV 18 seroconversion rate: 99.3% (95% CI, 98.4% to 100%) | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Injection site AE: RR, 1.12 (95% CI, 1.06 to 1.18); systemic AE: RR, 0.99 (95% CI, 0.91 to 1.1); no serious AE in either group |
| Ogawa and Colleagues, 201748x48Ogawa, Y., Takei, H., Ogawa, R., and Mihara, K. Safety of human papillomavirus vaccines in healthy young women: a meta-analysis of 24 controlled studies. J Pharm Health Care Sci. 2017; 3 Google ScholarSee all References | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Solicited local symptoms: RR, 1.20 (95% CI, 1.13 to 1.27); solicited systemic symptoms: RR, 1.04 (95% CI, 0.99 to 1.09); unsolicited symptoms: RR, 1.28 (95% CI, 1.01 to 1.63) compared with placebo but not different than hepatitis vaccine |
| Setiawan and Colleagues, 201752x52Setiawan, D., Luttjeboer, J., Pouwels, K.B., Wilschut, J.C., and Postma, M.J. Immunogenicity and safety of human papillomavirus (HPV) vaccination in Asian populations from six countries: a meta-analysis. Jpn J Clin Oncol. 2017; 47: 265–276 Google ScholarSee all References | HPV 16: uninfected population: 2vHPV RR, 44.86 (95% CI, 11.90 to 169.5); 4vHPV RR, 252.65 (95% CI, 35.77 to 1,784.59); combined RR, 62.52 (95% CI, 16.29 to 239.96); infected and uninfected populations RR, 8.6 (95% CI, 6.95 to 10.94); HPV 18: uninfected population: 2vHPV RR, 43.22 (95% CI, 25.52 to 73.68); 4vHPV RR, 96.04 (95% CI, 33.87 to 272.34); combined RR, 50.14 (95% CI, 31.17 to 50.68); infected and uninfected populations RR, 8.13 (95% CI, 5.96 to 11.11) | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Local AE: RR, 1.89 (95% CI, 1.65 to 2.17); systemic AE: RR, 1.33 (95% CI, 1.33 to 1.50) |
| Tejada and Colleagues, 201755x55Tejada, R.A., Vargas, K.G., Benites-Zapata, V., Mezones-Holguín, E., Bolaños-Díaz, R., and Hernandez, A.V. Human papillomavirus vaccine efficacy in the prevention of anogenital warts: systematic review and meta-analysis. Salud Publica Mex. 2017; 59: 84–94 Google ScholarSee all References | Not evaluated | Not evaluated | Not evaluated | Not evaluated | ITT: RR, 0.38 (95% CI, 0.32 to 0.45); per protocol: RR, 0.05 (95% CI, 0.01 to 0.25) | Not evaluated | Not evaluated | Not evaluated |
| Arbyn and Colleagues, 201830x30Arbyn, M., Xu, L., Simoens, C., and Martin-Hirsch, P.P.L. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018; 2018 Google ScholarSee all References | Not evaluated | HPV 16/18 incident infection: RR, 0.23 (95% CI, 0.14 to 0.37), GRADE: high | HPV 16/18 RR, 0.07 (95% CI, 0.05 to 0.09), GRADE: moderate; HPV 6/11/16/18 RR, 0.13 (95% CI, 0.05 to 0.37), GRADE: moderate | Not evaluated | Not evaluated | CIN 2+ associated with HPV 16/18 at 3-5 y: RR, 0.1 (95% CI, 0.0 to 0.05), GRADE: high; CIN 3+ associated with HPV16/18 at 3-5 y: RR, 0.01 (95% CI, 0.0 to 0.10), GRADE: high; adenocarcinoma in situ associated with HPV 16/18 at 3-5 y: RR, 0.10 (95% CI, 0.01 to 0.76), GRADE: moderate | Not evaluated | Serious AE at 6 mo to 7 y: RR, 0.98 (95% CI, 0.92 to 1.05), GRADE: high; death at 7 mo to 10 y: RR, 1.29 (95% CI, 0.85 to 1.98), GRADE: low |
| Harder and Colleagues, 201837x37Harder, T., Wichmann, O., Klug, S.J., van der Sande, M.A.B., and Wiese-Posselt, M. Efficacy, effectiveness and safety of vaccination against human papillomavirus in males: a systematic review. BMC Med. 2018; 16: 110–127 Google ScholarSee all References | Not evaluated | Not evaluated | Anogenital infection: Vaccine efficacy 46.9% (95% CI, 28.6 to 60.8), GRADE: moderate | Incident oral infection HPV 16 and/or 18: Vaccine efficacy 91% (95% CI, −59 to 99.5%), GRADE: very low; persisting oral infection: Vaccine efficacy 88% (95% CI, 2 to 98%), GRADE: moderate | Genital: Vaccine efficacy 89.4% (95% CI, 65.5 to 97.9%), GRADE: high; anal: Vaccine efficacy 100% (95% CI, 8.2 to 100%), GRADE: high | Anal intraepithelial neoplasia 2: Vaccine efficacy 61.9% (95% CI, 21.4 to 82.8), GRADE: moderate; anal intraepithelial neoplasia 3: Vaccine efficacy 46.8% (95% CI, –20 to 77.9), GRADE: low | Not evaluated | Not judged to be vaccine related but AE RR, 0.73 (95% CI, 0.25 to 1.99), GRADE: moderate |
| STUDY | SEROCONVERSION | HPV INCIDENT INFECTIONS | HPV PERSISTENT (> 6 MO) INFECTIONS | HPV ORAL INFECTIONS | HPV-RELATED WARTS | HPV EFFECTIVENESS | HPV-RELATED PRECANCEROUS LESIONS | HPV-RELATED ORAL CANCER | ADVERSE EVENTS |
|---|---|---|---|---|---|---|---|---|---|
| Agorastos and Colleagues, 200928x28Agorastos, T., Chatzigeorgiou, K., Brotherton, J.M., and Garland, S.M. Safety of human papillomavirus (HPV) vaccines: a review of the international experience so far. Vaccine. 2009; 27: 7270–7281 Google ScholarSee all References | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Risk difference between vaccinated and placebo group ranged from 9.4% (95% CI,† 7.3% to 11.5%) to 22.2% (95% CI, 19.7% to 24.7%) (local); 1.8% (95% CI, 0.3% to 3.2%) to 12.0% (95% CI, 10.8% to 13.2%) (systemic); 0% (95% CI, –0.1% to 0.1%) to 0.05% (95% CI, –0.03% to 0.1%) (serious). In surveillance data, 32 deaths (none judged likely to be caused by the vaccine) out of > 31.7 million vaccine doses |
| Yancey and Colleagues, 201057x57Yancey, A.M., Pitlick, J.M., and Forinash, A.B. The prophylactic role for the human papillomavirus quadrivalent vaccine in males. Annal Pharmacother. 2010; 44: 1314–1318 Google ScholarSee all References | Compared 4vHPV‡ vaccinated and 2vHPV vaccinated, 4vHPV geometric mean titers were noninferior to 2vHPV (P < .01) For 4vHPV, seroconversion rates were > 99.5% for HPV 6/11/16/18 at 1 month and > 92% at 1 y | Not evaluated | Compared vaccinated with placebo, risk difference of 40.9% | Not evaluated | Compared vaccinated with placebo, lower rate of condyloma: 10.6% (95% CI, 65.5% to 97.9%) | Not evaluated | Not evaluated | Not evaluated | Rate of 53.9 AEs per 100,000 doses, of which 6% were considered serious |
| Malagón and Colleagues, 201243x43Malagón, T., Drolet, M., Boily, M.C. et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012; 12: 781–789 Google ScholarSee all References | Not evaluated | Not evaluated | Compared vaccinated with non–HPV-vaccinated, vaccine efficacy against persistent infection with nonvaccine HPV types 31/33/45/52/58 ranged from –52.7% (95% CI, –883.5% to 70.3%) to 79.9% (95% CI, 61.3% to 89.4%) | Not evaluated | Not evaluated | Not evaluated | Compared vaccinated with non–HPV-vaccinated, vaccine efficacy against CIN# 2+ with nonvaccine HPV types 31/33/45/52/58 ranged from –132.3% (95% CI, –637.5% to 16.2%) to 100% (95% CI, 41.7% to 100%) | Not evaluated | Not evaluated |
| Macartney and Colleagues, 201341x41Macartney, K.K., Chiu, C., Georgousakis, M., and Brotherton, J.M.L. Safety of human papillomavirus vaccines: a review. Drug Saf. 2013; 36: 393–412 Google ScholarSee all References | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Risk differences between vaccinated and placebo group ranged from 0.5% to 34% (local); 0% to 12% (systemic); 0%, with no death judged related to the vaccine (serious). Inconsistent differences in local or systemic AEs by age; no statistically significant difference between males and females; higher rate of local AEs in 2vHPV vaccine compared with 4vHPV but no difference in systemic or serious AEs |
| Deleré and Colleagues, 201433x33Deleré, Y., Wichmann, O., Klug, S.J. et al. The efficacy and duration of vaccine protection against human papillomavirus. Dtsch Arztebl Int. 2014; 111: 584–591 () Google ScholarSee all References | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Compared vaccine era with prevaccine era, decrease in incidence of HPV infections or CIN 2+ lesions | Not evaluated | Not evaluated | Not evaluated |
| Garland and Colleagues, 201635x35Garland, S.M., Kjaer, S.K., Muñoz, N. et al. Impact and effectiveness of the quadrivalent human papillomavirus vaccine: a systematic review of 10 years of real-world experience. Clin Infect Dis. 2016; 63: 519–527 Google ScholarSee all References | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Compared vaccinated with at least 1 dose with unvaccinated, 76% to 89% decrease in prevalent HPV 6/11/16/18 infections Greatest decreases in those who received more than 1 dose 14%-88% decreases in prevalent HPV 16/18 in age cohort of those vaccinated compared with those in prevaccine era 45%-92.6% reduction in incidence of genital warts among those vaccinated compared with unvaccinated, with greater reduction in those vaccinated in youngest age groups 16%-60% declines in cervical cytologic abnormalities in those vaccinated compared with those unvaccinated Highest declines among those of younger ages when vaccinated or who received more than 1 dose | Not evaluated | Not evaluated | Not evaluated |
| Macki and Colleagues, 201642x42Macki, M. and Dabaja, A.A. Literature review of vaccine-related adverse events reported from HPV vaccination in randomized controlled trials. Basic Clin Androl. 2016; 26: 16 Google ScholarSee all References | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Risk differences between vaccinated and placebo group ranged from 1.7% to 27.8% (local); –13.7% to 29.6% (systemic); –0.6% to 0.6% (serious) |
| Martínez-Lavín and Colleagues, 201745x45Martínez-Lavín, M. and Amezcua-Guerra, L. Serious adverse events after HPV vaccination: a critical review of randomized trials and post-marketing case series. Clin Rheumatol. 2017; 36: 2169–2178 Google ScholarSee all References | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Risk difference between vaccinated and unvaccinated ranged from –0.9% to 8% (systemic); –1.2% to 5% (serious) Risk difference between 9vHPV and 4vHPV vaccine ranged from –4.5% to 3.9% (systemic); –0.5% to 0.7% (serious) |
| Signorelli and Colleagues, 201753x53Signorelli, C., Odone, A., Ciorba, V. et al. Human papillomavirus 9-valent vaccine for cancer prevention: a systematic review of the available evidence. Epidemiol Infect. 2017; 145: 1962–1982 Google ScholarSee all References | Compared 9vHPV vaccinated and 4vHPV vaccinated, statistically similar geometric mean titers for HPV 6/11/16/18 3 mo after participants vaccinated with 9vHPV, > 95% of participants seroconverted for HPV 31/33/45/52/58. | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Compared 9vHPV vaccinated with 4vHPV vaccinated, risk difference, 0.7 (95% CI, –15.7 to 14.8) | Not evaluated | Risk difference between 9vHPV and placebo was 4.5% (local); 4% (systemic); 0.5% (serious) Risk difference between 9vHPV and 4vHPV ranged from 3.3% to 7% (local); –4.5% to 0.9% (systemic); –0.4% to 0.7% (serious) |
| Harder and Colleagues, 201837x37Harder, T., Wichmann, O., Klug, S.J., van der Sande, M.A.B., and Wiese-Posselt, M. Efficacy, effectiveness and safety of vaccination against human papillomavirus in males: a systematic review. BMC Med. 2018; 16: 110–127 Google ScholarSee all References | Not evaluated | Compared vaccinated with placebo, vaccine efficacy against incident anogenital HPV 16: ranged from 28.0% (95% CI, 12.9% to 40.7%) to 45.1% (95% CI, 18.0% to 63.7%); HPV 18: ranged from 33.9% (95% CI, 13.0% to 50.1%) to 49.5% (95% CI, 11.3% to 72.1%) | Compared vaccinated with placebo, vaccine efficacy against persisting anogenital infection with HPV 16 ranged from 46.9% (95% CI, 28.6% to 60.8%) to 54% (95% CI, 23.9% to 72.9%); with HPV 18 ranged from 56.0% (95% CI, 28.8% to 73.7%) to 73.6% (95% CI, 37.5% to 90.3%) | Compared vaccinated with placebo, vaccine efficacy against persisting oral infection was 88% (95% CI, 2% to 98%); against incident oral infection with HPV 16/18 was 91% (95% CI, –59.0% to 99.5%) | Compared vaccinated with placebo, vaccine efficacy against condylomata acuminata ranged from –26% (95% CI, –130% to 31%) to 67.2% (95% CI, 47.3% to 80.3%) | Not evaluated | Compared vaccinated with placebo, vaccine efficacy against AIN†† 2+ ranged from 46.8% (95% CI, –20% to 77.9%) to 61.9% (95% CI, 21.4% to 82.8%) | Not evaluated | Risk difference between vaccinated and unvaccinated, –0.15 |
| Markowitz and Colleagues, 201844x44Markowitz, L.E., Drolet, M., Perez, N., Jit, M., and Brisson, M. Human papillomavirus vaccine effectiveness by number of doses: systematic review of data from national immunization programs. Vaccine. 2018; 36: 4806–4815 Google ScholarSee all References | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Compared vaccinated with unvaccinated: decreased aOR‡‡ of HPV 16/18 ranged from aOR, 0.27 (95% CI, 0.20 to 0.37) to aOR, 0.95 (95% CI, 0.51 to 1.76); decreased aRR for anogenital warts ranged from aRR, 0.12 (95% CI, CI 0.07 to 0.21) to aRR, 0.54 (95% CI, 0.46 to 0.56); aRR of CIN 3/adenocarcinoma in situ ranged from aRR, 0.45 (95% CI, 0.35 to 0.77) to aRR, 1.42 (95% CI, 0.89 to 2.28); decreased aOR of high grade histologic lesions ranged from aOR, 0.54 (95% CI, 0.43 to 0.67) to aOR, 0.95 (95% CI, 0.77 to 1.16); decreased change of abnormal cytology ranged from aOR, 0.17 (95% CI, 0.02 to 1.20) to aRR, 1.05 (95% CI, 0.88 to 1.26) | Not evaluated | Not evaluated | Not evaluated |
| Yakely and Colleagues, 201856x56Yakely, A.E., Avni-Singer, L., Oliveira, C.R., and Niccolai, L.M. Human papillomavirus vaccination and anogenital warts: a systematic review of impact and effectiveness in the United States. Sex Transm Dis. 2018; 46: 213–220 Google ScholarSee all References | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Not evaluated | Compared vaccinated with unvaccinated, reduced HR of anogenital warts ranged from HR, 0.23 (95% CI, 0.17 to 0.31) to HR, 0.91 (95% CI, 0.59 to 1.41). There was a dose-response relationship between increasing number of doses and decreased anogenital wart rate | Not evaluated | Not evaluated | Not evaluated |